A68300
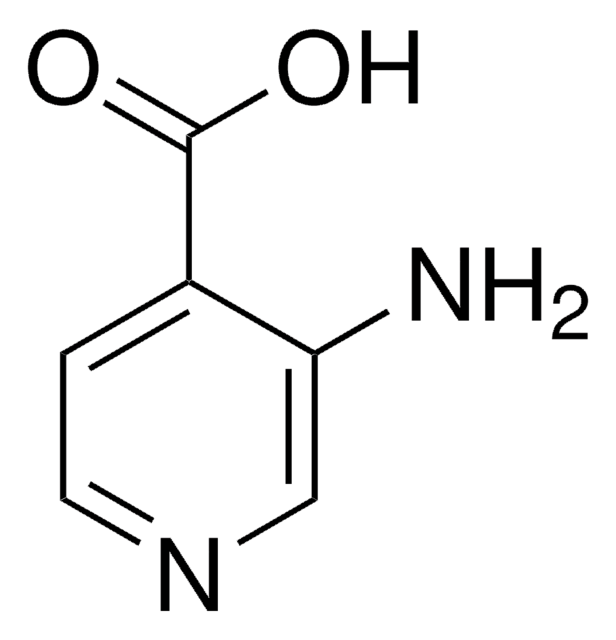
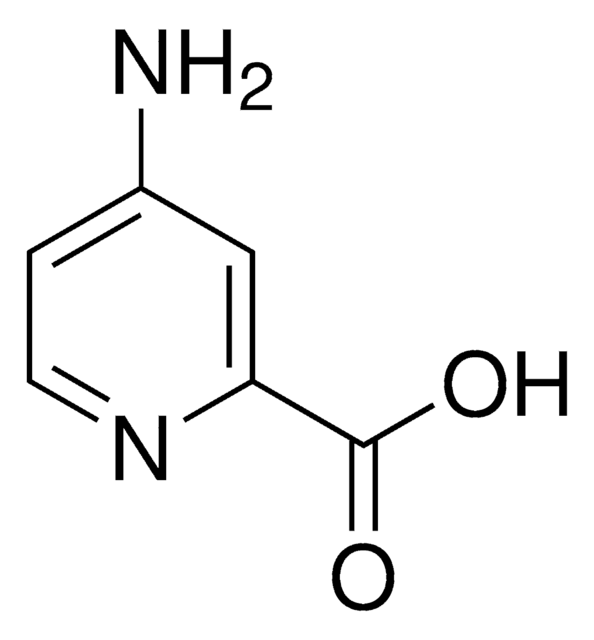
2-Aminopyridine-3-carboxylic acid
98%
Synonym(s):
2-Aminonicotinic acid, 2-Aminopyridine-3-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O2
CAS Number:
Molecular Weight:
138.12
Beilstein:
119031
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
295-297 °C (dec.) (lit.)
SMILES string
Nc1ncccc1C(O)=O
InChI
1S/C6H6N2O2/c7-5-4(6(9)10)2-1-3-8-5/h1-3H,(H2,7,8)(H,9,10)
InChI key
KPIVDNYJNOPGBE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Aminopyridine-3-carboxylic acid can be used as:
- A ligand to prepare copper(II)-organic coordination compounds.
- A reactant to prepare pyrido[2′,1′:2,3]imidazo[4,5-c]isoquinolines by reacting with trimethylsilyl cyanide and phthalaldehyde.
- A reactant to synthesize organo-soluble and thermally stable poly(thiourea-amide-imide) polymers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mehmet Karabacak et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 91, 83-96 (2012-03-01)
The experimental (UV-vis and FT-IR) and theoretical study of 2-aminonicotinic acid (C(6)H(6)N(2)O(2)) was presented in this work. The ultraviolet absorption spectrum of title molecule that dissolved in ethanol and water were examined in the range of 200-400 nm. The FT-IR
Synthesis, characterization, spectroscopic and electrochemical investigation of 2-aminopyridine-3-carboxylic acid copper (II) complexes with diimine
Srivastava AK, et al.
Chemical Data Collections, 24, 100272-100272 (2019)
Ronald Bartzatt
Drugs in R&D, 8(6), 363-372 (2007-10-30)
Nitrogen mustard (N-mustard) compounds are considered important anticancer drugs. Various transporting agents have been utilised to carry N-mustard groups including coumarins, amides, polyaromatic molecules and cycloalkyl structures. N-mustards act as bifunctional alkylating agents that induce cross-linking within DNA strands and
Synthesis of pyrido [2′, 1′: 2, 3] imidazo [4, 5-c] isoquinolines via a one-pot, three-component reaction
Maleki A and Rezayan AH
Tetrahedron Letters, 55(10), 1848-1850 (2014)
Novel thermally stable poly (thiourea-amide-imide) s bearing CS moieties and pyridine units in the backbone: Synthesis and properties
Kausar A, et al.
Polymer Degradation and Stability, 95(12), 2611-2618 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service