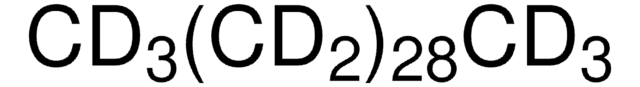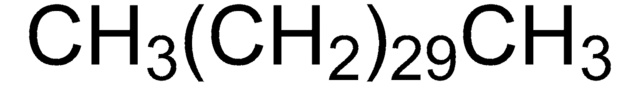All Photos(1)
About This Item
Linear Formula:
CH3(CH2)28CH3
CAS Number:
Molecular Weight:
422.81
Beilstein:
1777281
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
258-259 °C/3 mmHg (lit.)
mp
64-67 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCCCCCCCCCCCCCCC
InChI
1S/C30H62/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h3-30H2,1-2H3
InChI key
JXTPJDDICSTXJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Triacontane is a straight-chain alkane that can be produced from palmitic acid via Kolbe electrolysis.
Interfacial molecular ordering of triacontane, at the SiO2/air interface, for submonolayer and excess coverage has been investigated.
Interfacial molecular ordering of triacontane, at the SiO2/air interface, for submonolayer and excess coverage has been investigated.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Long chain n-alkanes at SiO2/air interfaces: Molecular ordering, annealing, and surface freezing of triacontane in the case of excess and submonolayer coverage.
Schollmeyer H, et al.
Langmuir, 19(12), 5042-5051 (2003)
Electrochemical conversion of palmitic acid via Kolbe electrolysis for synthesis of n-triacontane
Zhang Y, et al.
Journal of Electroanalytical Chemistry, 822, 73-80 (2018)
Scott A Harding et al.
Tree physiology, 34(11), 1240-1251 (2013-12-18)
The partitioning of carbon for growth, storage and constitutive chemical defenses is widely framed in terms of a hypothetical sink-source differential that varies with nutrient supply. According to this framework, phenolics accrual is passive and occurs in source leaves when
Pavani P Nadiminti et al.
Protoplasma, 252(6), 1475-1486 (2015-02-26)
Electron microscopy techniques such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM) have been invaluable tools for the study of the micromorphology of plant cuticles. However, for electron microscopy, the preparation techniques required may invariably introduce artefacts in
Luana Quassinti et al.
Fitoterapia, 97, 133-141 (2014-06-14)
Smyrnium olusatrum (Apiaceae), well known as wild celery, is a biennal celery-scented plant used for many centuries as a vegetable, then abandoned after the introduction of celery. In the present work, the essential oil obtained from inflorescences and the amounts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service