192171
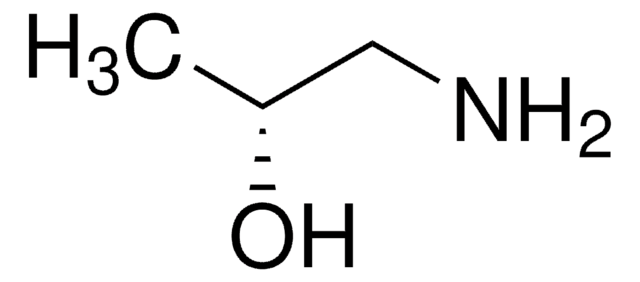
DL-Alaninol
98%
Synonym(s):
(±)-2-Amino-1-propanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(NH2)CH2OH
CAS Number:
Molecular Weight:
75.11
Beilstein:
1209234
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
optical activity
[α]/D +1.0 to −1.0°
refractive index
n20/D 1.450 (lit.)
bp
173-176 °C (lit.)
density
0.943 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
CC(N)CO
InChI
1S/C3H9NO/c1-3(4)2-5/h3,5H,2,4H2,1H3
InChI key
BKMMTJMQCTUHRP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
DL-Alaninol has been used in the synthesis of:
- N6-α-(I-hydroxypropyl) lysine
- diastereomers of DL-β-amino alcohols
- enantiopure and racemic samples of 2-methyl-N-tosylaziridine
- (±)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
183.2 °F - closed cup
Flash Point(C)
84 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ian C Stewart et al.
Journal of the American Chemical Society, 127(50), 17616-17617 (2005-12-15)
Sulfonylaziridines have been identified as excellent monomers for living ring-opening polymerization initiated by nucleophilic sulfonylamides. The resulting polymers exhibit low polydispersities and controllable molecular weights. The enantiopurity of the monomer plays a key role: racemic samples yield soluble polymers of
P M Hoi et al.
British journal of pharmacology, 152(5), 751-764 (2007-09-25)
A putative novel cannabinoid receptor mediates vasorelaxation to anandamide and abnormal-cannabidiol and is blocked by O-1918 and by high concentrations of rimonabant. This study investigates VSN16, a novel water-soluble agonist, as a vasorelaxant potentially acting at non-CB1, non-CB2 cannabinoid receptors
The role of lysine residues in the activity of 2-keto-3-deoxy-6-phosphogluconate aldolase.
J M Ingram et al.
The Journal of biological chemistry, 240(11), 4146-4151 (1965-11-01)
The substrate-dependent steric course of the ethanolamine ammonia-lyase reaction.
P Diziol et al.
European journal of biochemistry, 106(1), 211-224 (1980-05-01)
Julien Pytkowicz et al.
Organic & biomolecular chemistry, 8(20), 4540-4542 (2010-09-08)
Two efficient routes are reported for the synthesis of both enantiomers of trifluoroalaninol in enantiopure form. The first pathway involves a Strecker-type reaction performed from a chiral trifluoromethyloxazolidine (Fox). The second route, which is more direct, involves, as a key
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service