374733
2,6-Diisopropylaniline
97%
Synonym(s):
2,6-Bis(1-methylethyl)benzenamine, 2,6-Bis(propan-2-yl)aniline, 2,6-Diisopropylphenylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
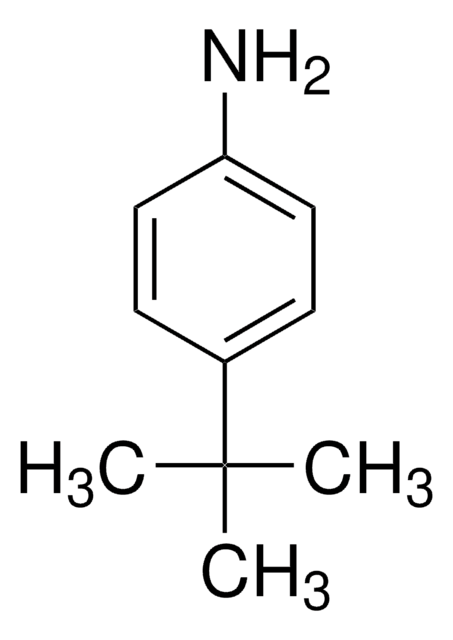
[(CH3)2CH]2C6H3NH2
CAS Number:
Molecular Weight:
177.29
Beilstein:
2208763
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
<0.01 mmHg ( 20 °C)
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.532 (lit.)
bp
257 °C (lit.)
mp
−45 °C (lit.)
density
0.94 g/mL at 25 °C (lit.)
SMILES string
CC(C)c1cccc(C(C)C)c1N
InChI
1S/C12H19N/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9H,13H2,1-4H3
InChI key
WKBALTUBRZPIPZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,6-Diisopropylaniline is an amine. It undergoes condensation with triacetylmethane in toluene in the presence of p-toluenesulfonic acid provides 3-[1-(2,6-diisopropylphenylamino)ethylidene]pentane-2,4-dione.
2,6-Diisopropylaniline is an important organic intermediate widely used to synthesize plastics and dyes.
2,6-Diisopropylaniline is an important organic intermediate widely used to synthesize plastics and dyes.
2,6-Diisopropylaniline is an aromatic amine. It reacts with bis(trimethylsilylmethyl)yttrium complexes supported by bulky amidopyridinate (Ap) and amidinate (Amd) ligands to afford yttrium alkyl anilido species. This reaction involves the elimination of TMS (Trimethylsilane).
2,6-Diisopropylaniline is an important organic intermediate widely used to synthesize plastics and dyes.
2,6-Diisopropylaniline is an important organic intermediate widely used to synthesize plastics and dyes.
Application
2,6-Diisopropylaniline may be used in the preparation of multitopic Schiff-base ligand precursors. It may be used in the preparation of NSN-donor proligand, 4,5-bis(2,6-diisopropylanilino)-2,7-di-tert-butyl-9,9-dimethylthioxanthene. It may be used to prepare N-heterocyclic carbene complexes for α-arylation of acyclic ketones, amination of haloarenes, and aqueous Suzuki coupling.
2,6-Diisopropylaniline may be used in the preparation of organocatalyst based on naphthalene diimides (NDIs).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electron-deficient naphthalene diimides as efficient planar Π-acid organocatalysts for selective oxidative C-C coupling of 2, 6-di-tert-butylphenol: A temperature effect.
Ke H, et al.
J. Mol. Catal. A: Chem., 385, 26-30 (2014)
Christoph Fleckenstein et al.
Chemical communications (Cambridge, England), (27), 2870-2872 (2007-07-05)
Sulfonated, water-soluble imidazolium and imidazolinium salts were synthesized and the respective Pd-complexes with N,N'-bis(2,6-dialkyl-4-SO(3)(-)-phenyl)imidazol-2-ylidene and N,N'-bis(2,6-dialkyl-4-SO(3)(-)-phenyl)-4,5-dihydroimidazol-2-ylidene ligands were applied in aqueous Suzuki coupling reactions of aryl chlorides.
Characterization and performance of Pd-La/spinel catalyst for preparation of 2, 6-diisopropylaniline
Ruixia J, et al.
Applied Catalysis A: General, 250(2), 209-220 (2003)
Kouki Matsubara et al.
The Journal of organic chemistry, 72(14), 5069-5076 (2007-06-15)
Arylation of both acyclic ketones and primary and secondary amines was achieved using a new, simple, stable, and easy-to-access nickel(II)-halide complex bearing mixed PPh3/N-heterocyclic carbene ligands as a catalyst precursor. Acyclic ketones were first arylated at the alpha-position with the
Reactions of Bis (alkyl) yttrium Complexes Supported by Bulky N, N Ligands with 2, 6-Diisopropylaniline and Phenylacetylene.
Karpov AV, et al.
Organometallics, 31(15), 5349-5357 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service