All Photos(1)
About This Item
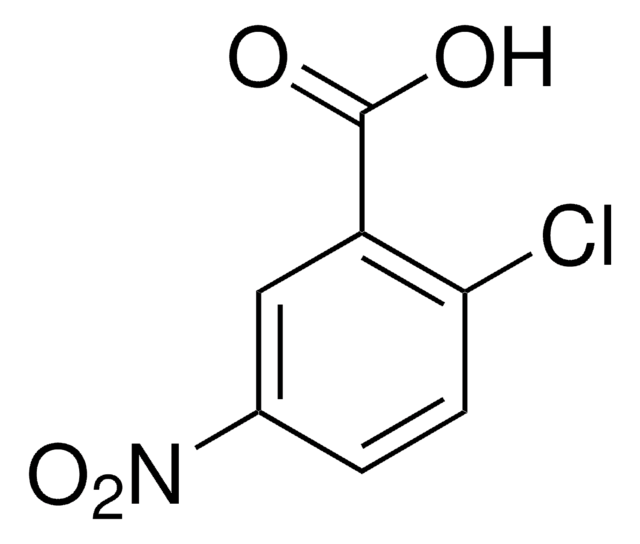
Linear Formula:
ClC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
201.56
Beilstein:
1877474
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
165-168 °C (lit.)
functional group
carboxylic acid
chloro
nitro
SMILES string
OC(=O)c1cc(ccc1Cl)[N+]([O-])=O
InChI
1S/C7H4ClNO4/c8-6-2-1-4(9(12)13)3-5(6)7(10)11/h1-3H,(H,10,11)
InChI key
QUEKGYQTRJVEQC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Chloro-5-nitrobenzoic acid undergoes microwave-assisted, regioselective amination reaction with aliphatic and aromatic amines to yield N-substituted 5-nitroanthranilic acid derivatives. It acts as ligand and forms a red luminescent one dimensional coordination polymer with Eu(III).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-Chloro-5-nitrobenzoato complexes of Eu (III) and Tb (III)-A 1D coordination polymer and enhanced solution luminescence.
Viswanathan S and Bettencourt-Dias A.
Inorganic Chemistry Communications, 9(5), 444-448 (2006)
Younis Baqi et al.
The Journal of organic chemistry, 72(15), 5908-5911 (2007-06-26)
The synthesis of N-substituted 5-nitroanthranilic acid derivatives 3a-w was achieved by a new, mild, microwave-assisted, regioselective amination reaction of 5-nitro-2-chlorobenzoic acid (1a) with a diverse range of aliphatic and aromatic amines 2a-w without added solvent or catalyst. Up to >99%
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service