All Photos(1)
About This Item
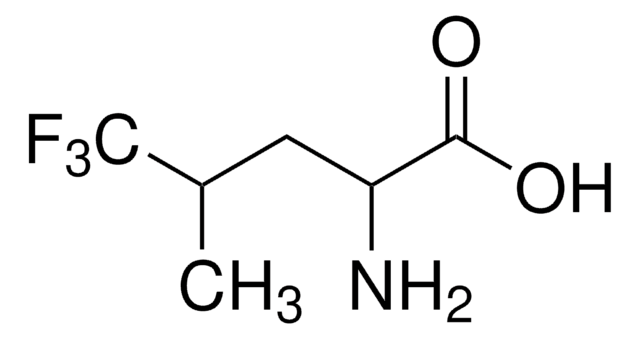
Linear Formula:
CF3CH(NH2)CO2H
CAS Number:
Molecular Weight:
143.06
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
reaction suitability
reaction type: solution phase peptide synthesis
mp
231-234 °C (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
NC(C(O)=O)C(F)(F)F
InChI
1S/C3H4F3NO2/c4-3(5,6)1(7)2(8)9/h1H,7H2,(H,8,9)
InChI key
HMJQKIDUCWWIBW-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W S Faraci et al.
Biochemistry, 28(2), 431-437 (1989-01-24)
The alanine racemases are a group of PLP-dependent bacterial enzymes that catalyze the racemization of alanine, providing D-alanine for cell wall synthesis. Inactivation of the alanine racemases from the Gram-negative organism Salmonella typhimurium and Gram-positive organism Bacillus stearothermophilus with beta
R S Phillips et al.
Archives of biochemistry and biophysics, 296(2), 489-496 (1992-08-01)
Trifluoroalanine is a mechanism-based inactivator of Escherichia coli tryptophan indole-lyase (tryptophanase) and E. coli tryptophan synthase (R. B. Silverman and R. H. Abeles, 1976, Biochemistry 15, 4718-4723). We have found that indole is able to prevent inactivation of tryptophan indole-lyase
C W Fearon et al.
Biochemistry, 21(16), 3790-3794 (1982-08-03)
Inactivation of gamma-cystathionase by beta, beta, beta-trifluoroalanine, a suicide inactivator of the enzyme, results in covalent labeling of an amino group of the protein [Silverman, R. B., & Abeles, R. H. (1977) Biochemistry 16, 5515-5520]. We have established that this
E A Wang et al.
Biochemistry, 20(26), 7539-7546 (1981-12-22)
The alanine racemase from Escherichia coli B has been shown to process DL isomers of beta -fluoroalanine as suicide substrates with an identical partitioning ratio for each enantiomer of 820 catalytic eliminations of HF per enzymatic inactivation event [Wang, E.
L Pollegioni et al.
FEBS letters, 507(3), 323-326 (2001-11-07)
D-Amino acid oxidase (DAAO) is a flavoprotein oxidase that catalyzes the oxidation of amino acids and produces ketoacids and H(2)O(2). The rate of product release from reduced DAAO from Rhodotorula gracilis is pH dependent and reflects a pK(a) of approximately
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service