568155
4,4,5,5,-Tetramethyl-2-phenylsulfanylmethyl-1,3,2-dioxaborolane
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
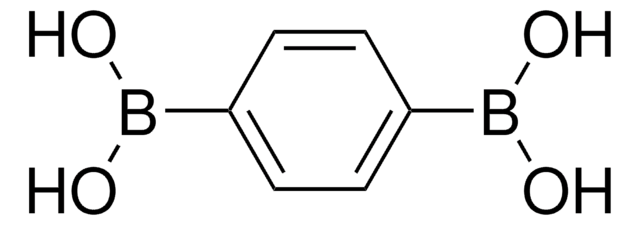
C6H5SCH2BC2O2(CH3)4
CAS Number:
Molecular Weight:
250.16
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.5280 (lit.)
bp
105-108 °C/0.1 mmHg (lit.)
density
1.059 g/mL at 25 °C (lit.)
SMILES string
CC1(C)OB(CSc2ccccc2)OC1(C)C
InChI
1S/C13H19BO2S/c1-12(2)13(3,4)16-14(15-12)10-17-11-8-6-5-7-9-11/h5-9H,10H2,1-4H3
InChI key
DGPGLPBMZOKGON-UHFFFAOYSA-N
Application
4,4,5,5,-Tetramethyl-2-phenylsulfanylmethyl-1,3,2-dioxaborolane is used to prepare aryl/heteroaryl derivatives via the formation of C-C and C-hetero bonds.
It can also be used as:
It can also be used as:
- A starting material in the synthesis of α-aminoboronic acids as serine proteases inhibitors.
- A substrate in the coupling reactions with carbonyl compounds under Ir-catalyzed photoredox conditions.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Photoredox Coupling Reaction of Benzylboronic Esters and Carbonyl Compounds in Batch and Flow
Chen Y, et al.
Organic Letters, 21(15), 6140-6144 (2019)
Synthesis of boronic acid analogues of α-amino acids by introducing side chains as electrophiles
Jagannathan S, et al.
The Journal of Organic Chemistry, 66(19), 6375-6380 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![Bis[(pinacolato)boryl]methane](/deepweb/assets/sigmaaldrich/product/structures/286/283/dcb13110-c536-4223-99e6-0dd505906b64/640/dcb13110-c536-4223-99e6-0dd505906b64.png)