473294
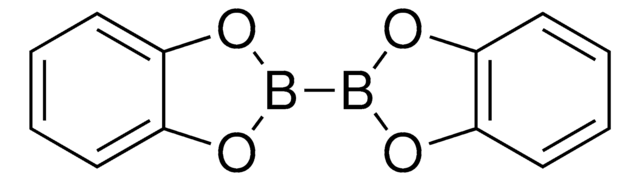
Bis(pinacolato)diboron
99%
Synonym(s):
4,4,4′,4′,5,5,5′,5′-Octamethyl-2,2′-bi-1,3,2-dioxaborolane
Sign Into View Organizational & Contract Pricing
All Photos(6)
About This Item
Empirical Formula (Hill Notation):
C12H24B2O4
CAS Number:
Molecular Weight:
253.94
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
grade:
for analytical purposes
form:
powder (or crystals)
Recommended Products
grade
for analytical purposes
Assay
99%
form
powder (or crystals)
mp
137-140 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)B2OC(C)(C)C(C)(C)O2
InChI
1S/C12H24B2O4/c1-9(2)10(3,4)16-13(15-9)14-17-11(5,6)12(7,8)18-14/h1-8H3
InChI key
IPWKHHSGDUIRAH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Bis(pinacolato)diboron or (B2pin2) is the most commonly used diborane reagent in organic synthesis due to its high stability in air and moisture. It can be synthesized by treating tetrakis(dimethylamino)diboron with pinacol in acidic conditions.
Application
Reagent used for the cis-vicinal diborylation of acetylenes and olefins with Pt catalysis; borylation of aromatics by Pd catalysis.
Substrate used in a new palladium-catalyzed cyclization of 1,6-enynes leading to homoallylic alkylboronates.
Used to construct a tetramethylpyrrolidine nitroxide scaffold for the synthesis of paramagnetic heterocycles by Suzuki coupling.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, 2573-2573 (2006)
Pd-catalyzed borylative cyclization of 1,6-enynes.
Juan Marco-Martínez et al.
Journal of the American Chemical Society, 129(7), 1874-1875 (2007-01-31)
Synlett, 15, 2442-2443 (2003)
Catalysis of the coupling reaction of aryl chlorides with bis (pinacolato) diboron by tricyclohexylphosphine-cyclopalladated ferrocenylimine complexes
Xu C, et al.
Transition Metal Chemistry, 34(2), 175-179 (2009)
Ronald L Reyes et al.
Science (New York, N.Y.), 369(6506), 970-974 (2020-08-21)
Site selectivity and stereocontrol remain major challenges in C-H bond functionalization chemistry, especially in linear aliphatic saturated hydrocarbon scaffolds. We report the highly enantioselective and site-selective catalytic borylation of remote C(sp3)-H bonds γ to the carbonyl group in aliphatic secondary
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)








![Bis[(pinacolato)boryl]methane](/deepweb/assets/sigmaaldrich/product/structures/286/283/dcb13110-c536-4223-99e6-0dd505906b64/640/dcb13110-c536-4223-99e6-0dd505906b64.png)




