362700
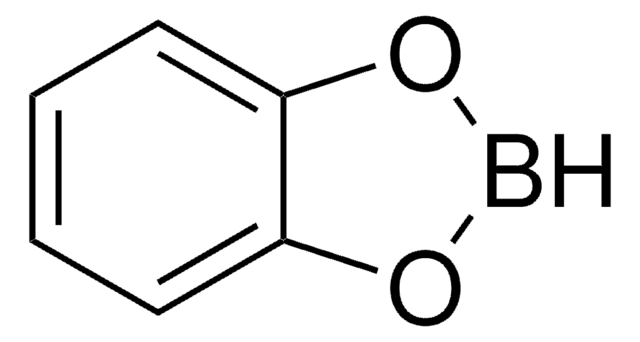
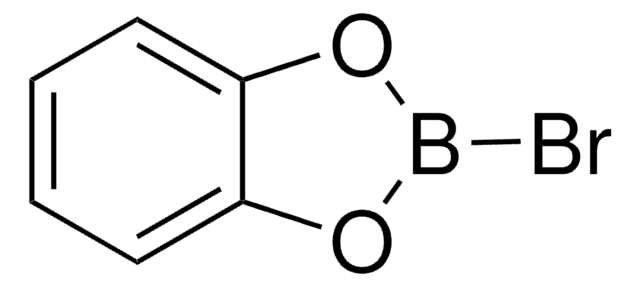
B-Chlorocatecholborane
97%
Synonym(s):
2-Chloro-1,3,2-benzodioxaborole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H4BClO2
CAS Number:
Molecular Weight:
154.36
Beilstein:
2093985
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
56-58 °C (lit.)
SMILES string
Clb1oc2ccccc2o1
InChI
1S/C6H4BClO2/c8-7-9-5-3-1-2-4-6(5)10-7/h1-4H
InChI key
AZYGEWXDKHFOKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
B-Chlorocatecholborane is a boron reagent and a Lewis acid, known to facilitate the borylative cyclization of alkynes to yield the borylated heterocycles. It is also used in the preparation of lactones, and thiophenes.
Application
B-Chlorocatecholborane can be used:
- To prepare 2-arachidonoylglycerol by acetal cleavage of cis-arachidonoylbenzylidene glycerol.
- To prepare metal boryl complexes (Rh and Ir complexes) through oxidative addition.
- To remove the trityl group in one of the key steps for the synthesis of (−)-dictyostatin.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
140.0 °F - closed cup
Flash Point(C)
60 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and biological evaluation of (−)-dictyostatin and stereoisomers
Shin Y, et al.
Tetrahedron, 63(35), 8537-8562 (2007)
Tetrahedron Letters, 26, 1411-1411 (1985)
Chao Gao et al.
The Journal of organic chemistry, 85(16), 10350-10368 (2020-07-17)
In contrast to previously reported borylative heterocyclization methods, a reaction here proceeds without air-free techniques to access synthetically useful borylated thiophenes, benzothiophenes, and isocoumarins. A comparison of stability/decomposition rates in air of several catecholboronic ester (Bcat) compounds derived from different
Mild acetal cleavage using B-chlorocatecholborane in the synthesis of rearrangement-sensitive 2-arachidonoylglycerol
Roche MJ, et al.
Tetrahedron Letters, 53(30), 3825-3827 (2012)
Synthesis of rhodium and iridium boryl complexes via oxidative addition of haloboranes
Souza FES, et al.
Inorgorganica Chimica Acta, 358(5), 1501-1509 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service