73032AST
Astec® CHIRALDEX™ G-TA Capillary GC Column
L × I.D. 20 m × 0.25 mm, df 0.12 μm
About This Item
Produits recommandés
Matériaux
fused silica
Niveau de qualité
Description
GC capillary column
Conditionnement
pkg of 1 ea
Paramètres
-10-180 °C temperature (isothermal or programmed)
Valeur bêta
500
df
0.12 μm
Technique(s)
gas chromatography (GC): suitable
L × D.I.
20 m × 0.25 mm
Groupe de la matrice active
non-bonded; 2,6-di-O-pentyl-3-trifluoroacetyl derivative of γ-cyclodextrin phase
Application(s)
agriculture
chemicals and industrial polymers
cleaning products
clinical
cosmetics
environmental
flavors and fragrances
food and beverages
forensics and toxicology
life science and biopharma
personal care
pharmaceutical (small molecule)
Type de colonne
capillary chiral
Technique de séparation
chiral
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Résistance chim./phys.
- -10 °C to 180 °C isothermal and programmed
Autres remarques
Informations légales
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
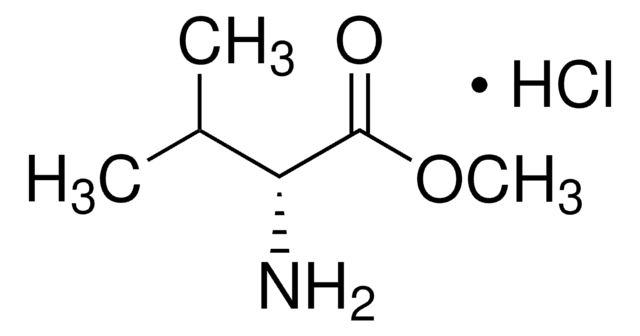
Chromatographic enantiomeric separation of amino acids, like proline, is described for chiral GC analysis after derivatization.
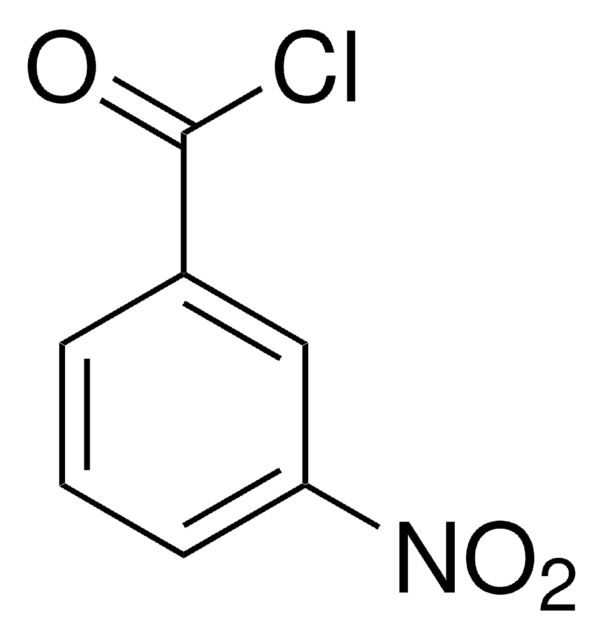
Chromatographic enantiomeric separation of amino acids, like proline, is described for chiral GC analysis after derivatization.
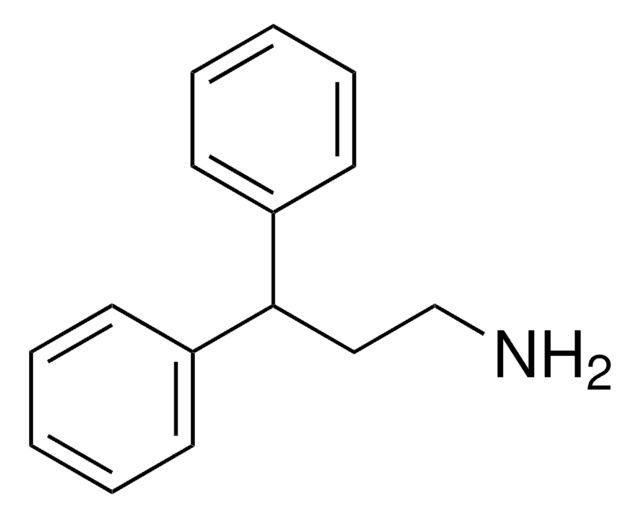
Chromatographic enantiomeric separation of amino acids, like proline, is described for chiral GC analysis after derivatization.
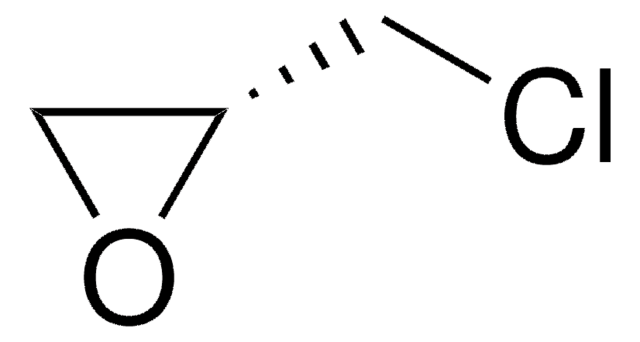
Chromatographic enantiomeric separation of amino acids, like proline, is described for chiral GC analysis after derivatization.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


![5-Boc-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridine-3-carboxylic acid](/deepweb/assets/sigmaaldrich/product/structures/145/700/2ac352d1-30ff-42c1-b2f3-0fe11cecf36f/640/2ac352d1-30ff-42c1-b2f3-0fe11cecf36f.png)