MAK172
Proteasome 20S Activity Assay Kit
sufficient for 100 fluorometric tests
Synonyme(s) :
20S Proteasome Detection Kit
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Utilisation
sufficient for 100 fluorometric tests
Méthode de détection
fluorometric
Maladie(s) pertinente(s)
endocrinological disorders, diabetes; neurological disorders; cancer
Température de stockage
−20°C
Catégories apparentées
Description générale
Protein degradation occurs most commonly through two major mechanisms, lysosomal degradation and proteasome mediated degradation. Lysosomal degradation occurs through proteolytic enzyme activity and is fairly non-specific. Proteasomal degradation is targeted for a specific protein through the ubiquitination of the target protein. The target protein is typically poly-ubiquitinated and activates the 26S proteasome toward proteolysis. The proteasome 26S is the most common form of the proteasome and is an ATP-dependent proteolytic complex, which contains one 20S (700-kDa) core particle structure and two 19S (700-kDa) regulatory caps.
The proteasome is responsible for the recycling of proteins and maintenance of the balance of protein synthesis and degradation. The proteasome is involved with apoptosis, DNA repair, endocytosis, and cell cycle and has been implicated in disease states such as certain types of cancer, diabetes mellitus, and Alzheimer′s disease among others making it the target for drug discovery investigations.
The proteasome is responsible for the recycling of proteins and maintenance of the balance of protein synthesis and degradation. The proteasome is involved with apoptosis, DNA repair, endocytosis, and cell cycle and has been implicated in disease states such as certain types of cancer, diabetes mellitus, and Alzheimer′s disease among others making it the target for drug discovery investigations.
Caractéristiques et avantages
Compatible with high-throughput handling systems.
Adéquation
Suitable for measuring the chymotrypsin-like protease activity associated with the proteasome complex either in cultured cells or cell lysates.
Principe
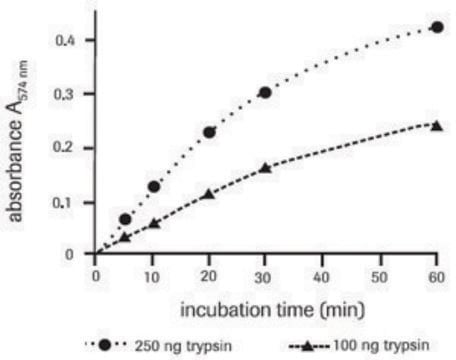
The fluorometric proteasome 20S assay kit is a homogeneous fluorescent assay that measures the chymotrypsin-like protease activity associated with the proteasome complex in cultured cells. This kit uses LLVY-R110 as a fluorogenic indicator for proteasome activities. Cleavage of LLVY-R110 by proteasome generates strongly green fluorescent R110 that is monitored fluorimetrically at 520-530 nm with excitation at 480-500 nm. The assay is robust, and can be readily adapted for high-throughput assays to evaluate the proteasome activities or screen the inhibitors in cultured cells or in solution. The assay can be performed in a convenient 96-well and 384-well fluorescence microtiter-plate format.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Javier Quero et al.
Antioxidants (Basel, Switzerland), 10(2) (2021-02-11)
The application of plant extracts for therapeutic purposes has been used in traditional medicine because plants contain bioactive compounds with beneficial properties for health. Currently, the use of these compounds that are rich in polyphenols for the treatment and prevention
Danielle M Drake et al.
Redox biology, 48, 102148-102148 (2021-11-05)
The breast cancer 1 protein (BRCA1) facilitates DNA repair, preventing embryolethality and protecting the fetus from reactive oxygen species (ROS)-induced developmental disorders mediated by oxidatively damaged DNA. Alcohol (ethanol, EtOH) exposure during pregnancy causes fetal alcohol spectrum disorders (FASD), characterized
The Cell: A Molecular Approach (2000)
The proteasome: Overview of structure and functions
Tanaka K
Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 85(1), 12-36 (2009)
Inés Mármol et al.
Inorganic chemistry, 59(23), 17732-17745 (2020-11-19)
A series of gold(I) and silver(I) derivatives with N- or S-donor ligands derived from 2-anilinopyridine has been synthesized and characterized. The mononuclear structure of [Au(L1)(PPh3)](TfO) (1a) and [Au(L2)(PPh3)](TfO) (1b) was confirmed by X-ray diffraction studies, as well as the dinuclear
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique