H4417
Human Collagen Type IV
from human placenta, liquid, High Performance
About This Item
Produits recommandés
product name
Collagène from human placenta, Bornstein and Traub Type IV, solution, suitable for cell culture, High Performance
Source biologique
human placenta
Niveau de qualité
Forme
solution
Technique(s)
cell culture | mammalian: suitable
Couverture de surface
<5 μg/cm2
Impuretés
Endotoxin, tested
HIV, hepatitis B and hepatitis C, none detected
Température de stockage
−20°C
Informations sur le gène
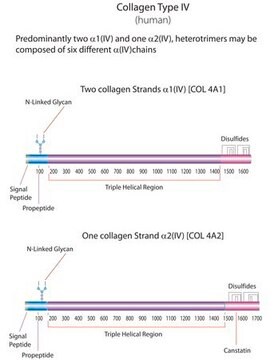
human ... COL4A1(1282) , COL4A2(1284) , COL4A3(1285) , COL4A4(1286) , COL4A5(1287) , COL4A6(1288)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Actions biochimiques/physiologiques
Tissue injury in the autoimmune disease Goodpasture syndrome is due to pathogenic autoantibodies targeting the Collagen IV α3 chain . Mutations in COL4A5 are associated with Alport syndrome.
Composants
Attention
Notes préparatoires
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique