F9813
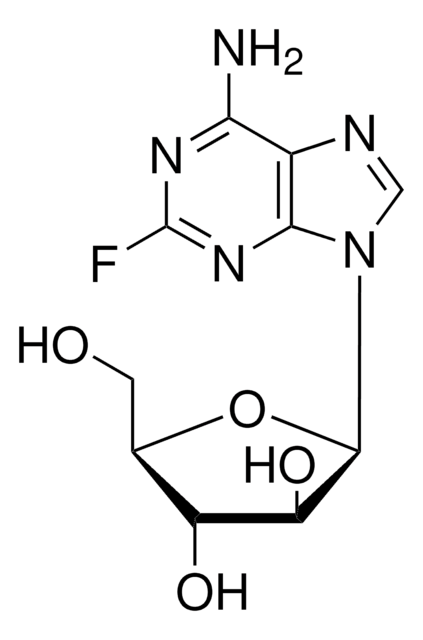
Fludarabine phosphate
Synonyme(s) :
2-Fluoro-9-(5-O-phosphono-β-D-arabinofuranosyl)-9H-purin-6-amine
About This Item
Produits recommandés
Forme
powder
Niveau de qualité
Couleur
white
Solubilité
DMSO: soluble
Spectre d'activité de l'antibiotique
neoplastics
Mode d’action
DNA synthesis | interferes
Température de stockage
−20°C
InChI
1S/C10H13FN5O7P/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,14,15)(H2,19,20,21)
Clé InChI
GIUYCYHIANZCFB-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Characterization of Chemical Interactions between Clinical Drugs and the Oral Bacterium, Corynebacterium matruchotii, via Bioactivity-HiTES.: This study explores the interactions of clinical drugs like Fludarabine phosphate with Corynebacterium matruchotii, highlighting potential impacts on oral microbiota and implications for drug efficacy and safety (Lee DY et al., 2024).
- Cocktail of lipophilic and hydrophilic chemotherapeutics in high-load core@shell nanocarriers to treat pancreatic tumours.: Investigates the efficacy of a combination of Fludarabine phosphate with other chemotherapeutics delivered via nanocarriers, aiming to enhance treatment outcomes for pancreatic cancer by improving drug delivery to the tumor site (Rudolph D et al., 2024).
- Macrophage neogenin deficiency exacerbates myocardial remodeling and inflammation after acute myocardial infarction through JAK1-STAT1 signaling.: This research demonstrates the role of Fludarabine phosphate in modulating inflammation and cardiac repair post-myocardial infarction, offering insights into its potential therapeutic benefits beyond oncology (Zhang J et al., 2023).
- SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis.: Discusses the cellular mechanisms by which Fludarabine phosphate may influence metabolic pathways in cancer cells, highlighting its potential to disrupt tumor metabolism and promote cancer cell death (Li Y et al., 2023).
- CD19-Targeting CAR T Cells for Myositis and Interstitial Lung Disease Associated With Antisynthetase Syndrome.: Reviews the use of Fludarabine phosphate in preconditioning regimens for CAR T-cell therapy, emphasizing its role in enhancing the efficacy of immunotherapy in treating autoimmune disorders (Pecher AC et al., 2023).
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Muta. 2 - Repr. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique