Description générale
Nuclear RNA export factor 1 (NXF1, TAP) is a nuclear RNA exporter protein that contains a noncanonical RNP-type RNA binding domain (RBD), 4 leucine-rich repeats (LRRs), a nuclear transport factor 2 (NTF2)-like domain that allows heterodimerization with NTF2-related export protein-1 (NXT1), and a ubiquitin-associated domain that mediates interactions with nucleoporins. Nuclear RNA export factor 1 exports poly(A)+RNA from the nucleus into the cytoplasm.
Spécificité
Anti-NXF1 polyclonal antibody reacts with canine, mouse, rat, human, and bovine nuclear RNA export factor 1 proteins.
Immunogène
Synthetic peptide directed towards the N terminal region of human NXF1
Application
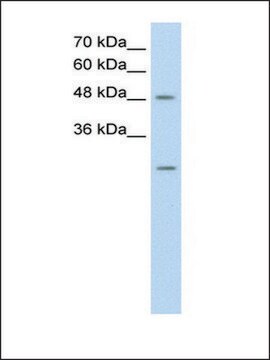
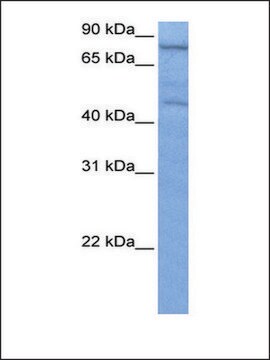
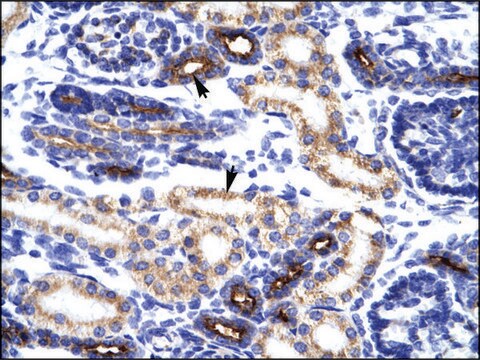
Anti-NXF1 polyclonal antibody is used to tag nuclear RNA export factor 1 protein for detection and quantitation by Western blotting and in plasma by immunohistochemical (IHC) techniques. It is used as a probe to determine the roles of nuclear RNA export factor 1 in polyA-RNA nuclear export.
Actions biochimiques/physiologiques
NXF1 is one member of a family of nuclear RNA export factor. Common domain features of this family are a noncanonical RNP-type RNA-binding domain (RBD), 4 leucine-rich repeats (LRRs), a nuclear transport factor 2 (NTF2)-like domain that allows heterodimerization with NTF2-related export protein-1 (NXT1), and a ubiquitin-associated domain that mediates interactions with nucleoporins. The LRRs and NTF2-like domains are required for export activity. NXF1 shuttles between the nucleus and the cytoplasm and binds in vivo to poly(A)+ RNA. NXF1 overcomes the mRNA export block caused by the presence of saturating amounts of CTE (constitutive transport element) RNA of type D retroviruses.This gene is one member of a family of nuclear RNA export factor genes. Common domain features of this family are a noncanonical RNP-type RNA-binding domain (RBD), 4 leucine-rich repeats (LRRs), a nuclear transport factor 2 (NTF2)-like domain that allows heterodimerization with NTF2-related export protein-1 (NXT1), and a ubiquitin-associated domain that mediates interactions with nucleoporins. The LRRs and NTF2-like domains are required for export activity. Alternative splicing seems to be a common mechanism in this gene family. The encoded protein of this gene shuttles between the nucleus and the cytoplasm and binds in vivo to poly(A)+ RNA. It is the vertebrate homologue of the yeast protein Mex67p. The encoded protein overcomes the mRNA export block caused by the presence of saturating amounts of CTE (constitutive transport element) RNA of type D retroviruses.This gene is one member of a family of nuclear RNA export factor genes. Common domain features of this family are a noncanonical RNP-type RNA-binding domain (RBD), 4 leucine-rich repeats (LRRs), a nuclear transport factor 2 (NTF2)-like domain that allows heterodimerization with NTF2-related export protein-1 (NXT1), and a ubiquitin-associated domain that mediates interactions with nucleoporins. The LRRs and NTF2-like domains are required for export activity. Alternative splicing seems to be a common mechanism in this gene family. The encoded protein of this gene shuttles between the nucleus and the cytoplasm and binds in vivo to poly(A)+ RNA. It is the vertebrate homologue of the yeast protein Mex67p. The encoded protein overcomes the mRNA export block caused by the presence of saturating amounts of CTE (constitutive transport element) RNA of type D retroviruses.
Séquence
Synthetic peptide located within the following region: RPNRRGDTWHDRDRIHVTVRRDRAPPERGGAGTSQDGTSKNWFKITIPYG
Forme physique
Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and 2% sucrose.
Clause de non-responsabilité
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.