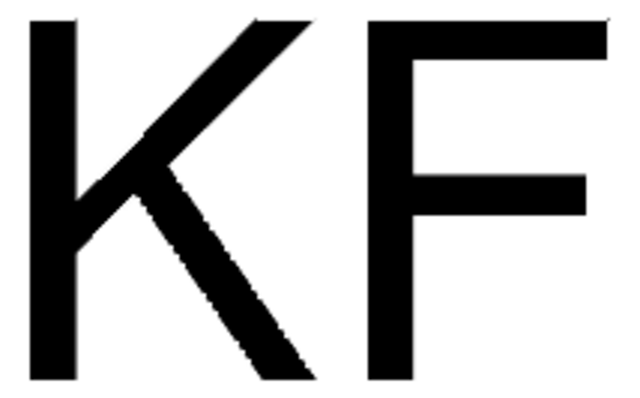939005
Cesium Fluoride ChemBeads
NSC 84270
Synonyme(s) :
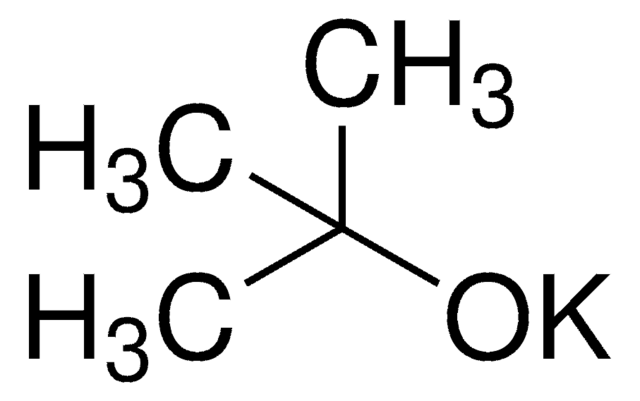
CsF
About This Item
Produits recommandés
Niveau de qualité
Forme
solid
Composition
, 14-16 wt. % (loading of base)
Chaîne SMILES
[F-].[Cs+]
InChI
1S/Cs.FH/h;1H/q+1;/p-1
Clé InChI
XJHCXCQVJFPJIK-UHFFFAOYSA-M
Description générale
Application
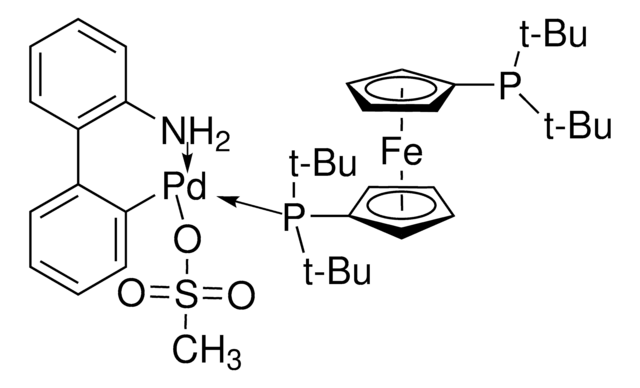
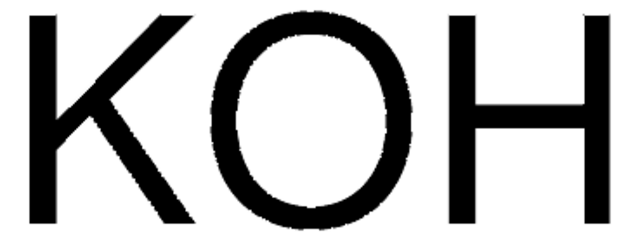
A base in the Suzuki cross-coupling synthesis of ortho-substituted biaryls.
A reagent for the nucleophilic fluorination of primary halides and sulfonates in protic media such as tert-butyl and tert-pentyl alcohols.
Reactant for:
Preparation of building blocks for synthesis of fluoroallylic compounds
Synthesis of alcohols via hydrolysis of alkyl silyl ethers at neutral pH in buffered mixed organic-aqueous solutions
Nucleophilic fluorination of alkynyliodonium salts to form fluorovinylic compounds
Nucleophilic aromatic substitution (SNAr) reactions
Used in the successful synthesis of a single-crystal Dion-Jacobson phase, CsLaTa2O7, that has applications in photocatalysis and superconductivity.
For general uses, product is also available in powdered form (198323)
Caractéristiques et avantages
Autres remarques
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Dam. 1 - Repr. 2 - STOT RE 2
Organes cibles
Kidney,Adrenal gland
Risques supp
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique