P55805
Purine
98%
Synonyme(s) :
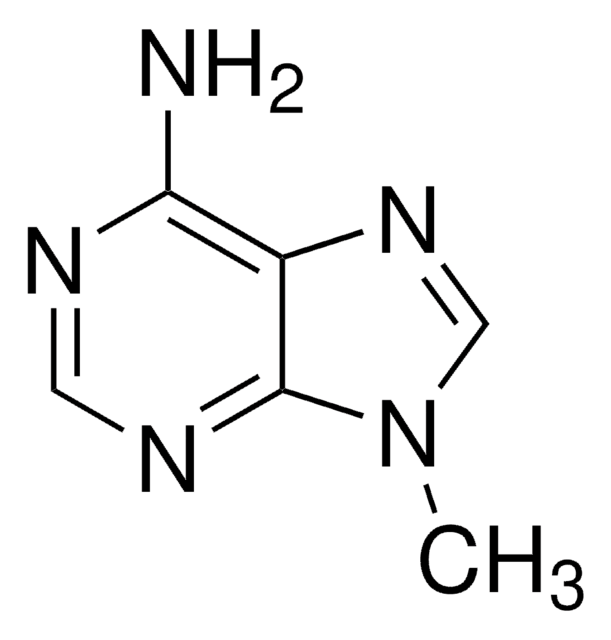
7H-Imidazo(4,5-d)pyrimidine
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C5H4N4
Numéro CAS:
Poids moléculaire :
120.11
Beilstein:
3200
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352005
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Essai
98%
Forme
powder
Pf
214-217 °C (lit.)
Chaîne SMILES
c1ncc2nc[nH]c2n1
InChI
1S/C5H4N4/c1-4-5(8-2-6-1)9-3-7-4/h1-3H,(H,6,7,8,9)
Clé InChI
KDCGOANMDULRCW-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Purine is a heterocyclic organic compound constituting a pyrimidine ring fused to an imidazole ring.
Application
Purine can undergo direct C-H functionalization in the presence of palladium catalyst to form various biologically important products.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
New developments in direct functionalization of C-H and N-H bonds of purine bases via metal catalyzed cross-coupling reactions.
Abdoli M, et al.
Royal Society of Chemistry Advances, 55(5), 44371-44389 (2015)
Benshang Li et al.
Nature medicine, 21(6), 563-571 (2015-05-12)
Relapse is the leading cause of mortality in children with acute lymphoblastic leukemia (ALL). Among chemotherapeutics, thiopurines are key drugs in ALL combination therapy. Using whole-exome sequencing, we identified relapse-specific mutations in the phosphoribosyl pyrophosphate synthetase 1 gene (PRPS1), which
Silvia Meneghesso et al.
ChemMedChem, 8(3), 415-425 (2013-02-07)
2'-Fluoro-2'-deoxyguanosine has been reported to have potent anti-influenza virus activity in vitro and in vivo. Herein we describe the synthesis and biological evaluation of 6-modified 2'-fluoro-2'-deoxyguanosine analogues and their corresponding phosphoramidate ProTides as potential anti-influenza virus agents. Whereas the parent
Hiroshi Ashihara et al.
Phytochemistry, 69(4), 841-856 (2007-12-11)
Details of the recently elucidated biosynthetic pathways of caffeine and related purine alkaloids are reviewed. The main caffeine biosynthetic pathway is a sequence consisting of xanthosine-->7-methylxanthosine-->7-methylxanthine-->theobromine-->caffeine. Genes encoding N-methyltransferases involved in three of these four reactions have been isolated and
Kansuporn Sriyudthsak et al.
BMC systems biology, 8 Suppl 5, S4-S4 (2015-01-07)
Progress in systems biology offers sophisticated approaches toward a comprehensive understanding of biological systems. Yet, computational analyses are held back due to difficulties in determining suitable model parameter values from experimental data which naturally are subject to biological fluctuations. The
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique