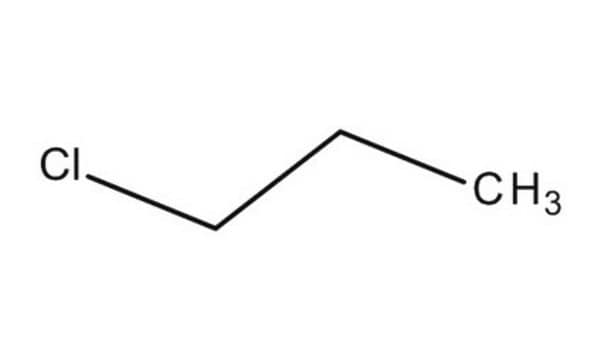
C68555
1-Chloropropane
98%
Synonyme(s) :
Propyl chloride
About This Item
Produits recommandés
Densité de vapeur
2.71 (vs air)
Niveau de qualité
Pression de vapeur
5.51 psi ( 20 °C)
Pureté
98%
Forme
liquid
Température d'inflammation spontanée
968 °F
Limite d'explosivité
11 %
Indice de réfraction
n20/D 1.388 (lit.)
Point d'ébullition
46-47 °C (lit.)
Pf
−123 °C (lit.)
Densité
0.892 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
CCCCl
InChI
1S/C3H7Cl/c1-2-3-4/h2-3H2,1H3
Clé InChI
SNMVRZFUUCLYTO-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- Theoretical and experimental studies of the kinetics of the reaction of 1-chloropropane and fully deuterated 1-chloropropane with atomic chlorine: This study explores the reaction kinetics of 1-chloropropane with atomic chlorine, providing insights into the influence of carbon position in molecular interactions (L Fojcik et al., 2021).
- High-pressure phase equilibrium in the {carbon dioxide (1)+ 1-chloropropane (2)} binary system: Research determining the phase equilibrium properties of 1-chloropropane under various conditions, useful for industrial applications involving carbon dioxide and 1-chloropropane (M Chorazewski et al., 2015).
- Temperature dependent dielectric relaxation studies of halopropane from 10 Mhz to 50 Ghz using a time domain reflectometry (TDR): This study investigates the dielectric properties of 1-chloropropane across a broad frequency range, highlighting its applications in the pharmaceutical and pesticide industries (RV Shinde et al., 2020).
- A Study on Subchronic Inhalation Toxicity of 1-Chloropropane: An examination of the inhalation toxicity of 1-chloropropane, providing critical data for occupational safety and health assessments (Y Hyun Chung et al., 2015).
- Thermal decomposition of 1-chloropropane behind the reflected shock waves in the temperature range of 1015–1220 K: A comprehensive analysis of the decomposition mechanisms of 1-chloropropane under high-temperature conditions, significant for understanding thermal stability and reaction kinetics (G Sudhakar et al., 2014).
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 2
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
-0.4 °F - closed cup
Point d'éclair (°C)
-18 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique