ALD00500
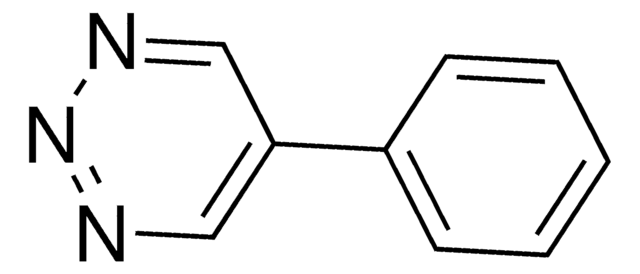
5-Methoxy-1,2,3-triazine
About This Item
Produits recommandés
Forme
powder
Niveau de qualité
Température de stockage
2-8°C
Chaîne SMILES
COC1=CN=NN=C1
InChI
1S/C4H5N3O/c1-8-4-2-5-7-6-3-4/h2-3H,1H3
Clé InChI
HVBZCUMRMKODNE-UHFFFAOYSA-N
Description générale
Application
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Contenu apparenté
As the exploration of the properties of complex natural products becomes increasingly more sophisticated with the technological advances being made in their screening and evaluation and as structural details of their interaction with biological targets becomes more accessible, the importance and opportunities for providing unique solutions to complex biological problems has grown. The Boger Lab addresses these challenging problems by understanding the complex solutions and subtle design elements that nature has provided in the form of a natural product and work to extend the solution through rational design elements to provide more selective, more efficacious, or more potent agents designed specifically for the problem or target under investigation. The resulting efforts have reduced many difficult or intractable synthetic challenges to manageable problems providing an approach not only to the natural product but one capable of simple extrapolation to a series of structural analogs with improved selectivity and efficacy.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique