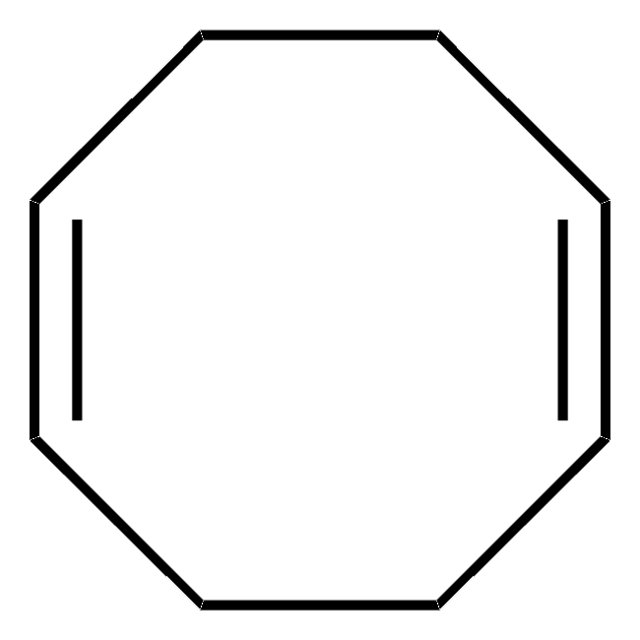
927090
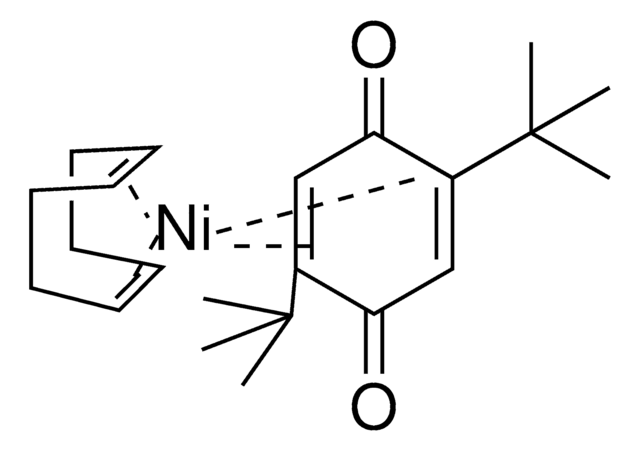
Ni(COD)(CPDO-(OMe)Ph)
≥95%
Synonyme(s) :
(1,5-cyclooctadiene)(2,3,4,5-tetrakis(4-methoxyphenyl)cyclopenta-2,4-dien-1-one)nickel(0)
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥95%
Forme
powder or chunks
Pertinence de la réaction
reagent type: catalyst
reaction type: Cross Couplings
Pf
>300 °C
Température de stockage
2-8°C
Application
Reference
Structurally Diverse Bench-Stable Nickel(0) Pre-Catalysts: A Practical Toolkit for In Situ Ligation Protocols
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Contenu apparenté
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique