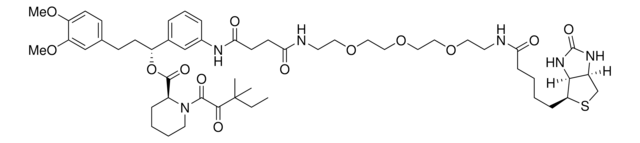
914975
KB03-SLF
≥95%
Synonyme(s) :
(R)-1-(3-(1-(3-(2-Chloroacetamido)-5-(trifluoromethyl)phenyl)-1,5-dioxo-9,12-dioxa-2,6-diazapentadecan-15-amido)phenyl)-3-(3,4-dimethoxyphenyl)propyl (S)-1-(3,3-dimethyl-2-oxopentanoyl)piperidine-2-carboxylate, Electrophilic PROTAC®, Heterobifunctional conjugate for E3 ubiquitin ligase discovery
About This Item
Produits recommandés
ligand
electrophilic fragment
Niveau de qualité
Pureté
≥95%
Forme
powder
Température de stockage
−20°C
Chaîne SMILES
ClCC(NC1=CC(C(NCCC(NCCOCCOCCC(NC2=CC=CC([C@H](OC([C@@H]3CCCCN3C(C(C(C)(C)CC)=O)=O)=O)CCC4=CC(OC)=C(OC)C=C4)=C2)=O)=O)=O)=CC(C(F)(F)F)=C1)=O
Catégories apparentées
Application
This proteomic approach to E3 discovery was demonstrated by Zhang et al in the discovery that DCAF16 mediated nuclear FKBP12 degradation via KB02-SLF.
Additional electrophilic PROTACs were developed incorporating scout fragments with broad cysteine reactivity:
- KB02-SLF (914738) containing chloroacetamide scout fragment 912131
- KB05-SLF (913715) containing acrylamide scout fragment 911798
Related tools:
- Additional bifunctional tools for FKPB12 variants: dTAG-13 (SML2601 for FKBP12F36V) and Biotin-SLF (914223 for FKBP12)
- Inhibitors useful in validation of proteasomal-mediated degradation: MG123 (SML1135) and MLN4924 (5.05477 for Cullin-RING ubiquitin ligases that regulate neddylation of Cullin proteins)
- Cereblon (CRBN) affinity probe: Biotin-Thalidomide (913979)
Autres remarques
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![(R)-2-(5-Bromo-4-(4-chlorobenzyl)-7-fluoro-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid](/deepweb/assets/sigmaaldrich/product/structures/321/793/9c59bf88-c483-4a85-a559-53ba29e916d1/640/9c59bf88-c483-4a85-a559-53ba29e916d1.png)


![[3-(2-carboxyethyl)phenyl]boronic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/265/067/68263dcf-5afc-49a6-982b-0394e48bf9c2/640/68263dcf-5afc-49a6-982b-0394e48bf9c2.png)