718149
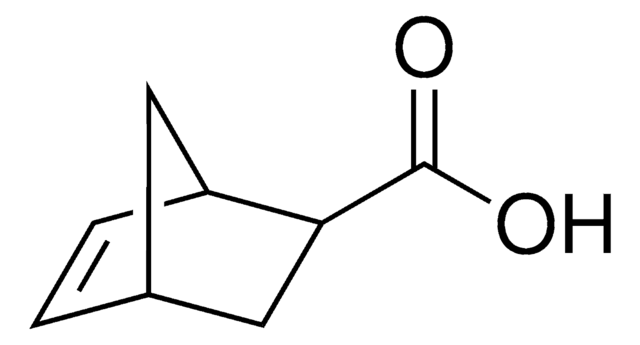
exo-5-Norbornenecarboxylic acid
97%
Synonyme(s) :
(1R,2S,4R)-Bicyclo[2.2.1]hept-5-ene-2-carboxylic acid, NC
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C8H10O2
Numéro CAS:
Poids moléculaire :
138.16
Numéro MDL:
Code UNSPSC :
12162002
ID de substance PubChem :
Nomenclature NACRES :
NA.23
Produits recommandés
Niveau de qualité
Pureté
97%
Forme
solid
Pf
40-44 °C
Chaîne SMILES
OC(=O)[C@@H]1C[C@@H]2C[C@H]1C=C2
InChI
1S/C8H10O2/c9-8(10)7-4-5-1-2-6(7)3-5/h1-2,5-7H,3-4H2,(H,9,10)/t5-,6+,7+/m0/s1
Clé InChI
FYGUSUBEMUKACF-RRKCRQDMSA-N
Description générale
Exo-5-norbornenecarboxylic acid is a bicyclic compound that has potential applications in the field of material science due to its unique chemical properties. It is a versatile building block for the synthesis of various functional materials, including polymers, dendrimers, and self-assembled monolayers. It can be used to modify surfaces or to functionalize nanoparticles, influencing their optical, magnetic, or electronic properties. It is also often used in ring-opening metathesis polymerization reactions to form polymers with controlled molecular weight and structure. Additionally, exo-5-norbornenecarboxylic acid can function as a ligand for coordination chemistry and catalysis.
Application
Exo-5-norbornenecarboxylic acid can be used as:
- A starting material in the synthesis of the metathesis polymer via a ring-opening metathesis polymerization (ROMP) reaction of the ester of exo-5-norbornenecarboxylic acid and 1,1′-bi-2-naphthol.
- A monomer in the preparation of thin films via surface-initiated polymerization process. The resulting thin film serves as a template for selective deposition and etching of metal oxides, which is of significant importance in the microelectronic industry.
- Different crosslinkers for ring-opening metathesis polymerization.
- Norbornene-functionalized monomers, which are used to make poly(norbornene)s via ring-opening metathesis polymerization (ROMP).
- Hydrolytically cleavable and hydrolytically stable functionalized macromonomers for hydrogel preparation.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
>230.0 °F
Point d'éclair (°C)
> 110 °C
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Jürgen Herrler et al.
Magnetic resonance in medicine, 85(6), 3140-3153 (2021-01-06)
To mitigate spatial flip angle (FA) variations under strict specific absorption rate (SAR) constraints for ultra-high field MRI using a combination of universal parallel transmit (pTx) pulses and fast subject-specific optimization. Data sets consisting of B0 , B 1 +
Wenjun Zheng et al.
BMC structural biology, 9, 45-45 (2009-07-14)
It is increasingly recognized that protein functions often require intricate conformational dynamics, which involves a network of key amino acid residues that couple spatially separated functional sites. Tremendous efforts have been made to identify these key residues by experimental and
Christopher L McGann et al.
Macromolecular bioscience, 16(1), 129-138 (2015-10-06)
A range of chemical strategies have been used for crosslinking recombinant polypeptide hydrogels, although only a few have employed photocrosslinking approaches. Here, we capitalize on the novel insect protein, resilin, and the versatility of click reactions to introduce a resilin-like
Patricia Gumbley et al.
Macromolecular rapid communications, 34(23-24), 1838-1843 (2013-11-12)
This communication describes photoresponsive gels, prepared using ring-opening metathesis polymerization (ROMP), that dissolve upon irradiation with ultraviolet light. Exposure of mixtures of norbornene-type ROMP monomers and new photoreactive cross-linkers comprising two norbornene units bound through a chain containing o-nitrobenzyl esters
Stefano Longhi et al.
Optics letters, 45(7), 1962-1965 (2020-04-03)
Multimode interference (MMI) and self-imaging are important phenomena of diffractive optics with major applications in signal processing, beam shaping, and optical sensing. Such phenomena generally arise from interference of normal modes in lossless dielectric guiding structures; however, the impact of
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique