473723
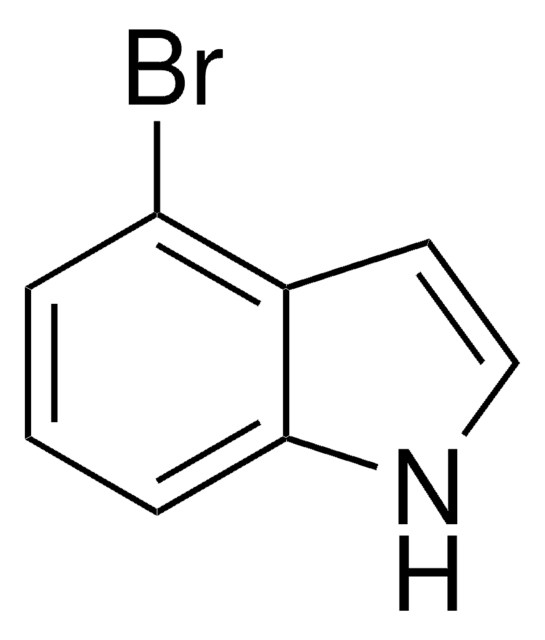
7-Bromoindole
96%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C8H6BrN
Numéro CAS:
Poids moléculaire :
196.04
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
96%
Forme
solid
Pf
41-44 °C (lit.)
Groupe fonctionnel
bromo
Chaîne SMILES
Brc1cccc2cc[nH]c12
InChI
1S/C8H6BrN/c9-7-3-1-2-6-4-5-10-8(6)7/h1-5,10H
Clé InChI
RDSVSEFWZUWZHW-UHFFFAOYSA-N
Description générale
7-Bromoindole is a 7-substituted indole derivative. Its synthesis from 7-bromoindole-2-carboxylic acid has been reported. It has been reported to reduce the production of staphyloxanthin in Staphylococcus aureus.
Application
7-Bromoindole may be used in the synthesis of the following:
- indole
- dyestuffs
- 8-bromocarboline
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
235.4 °F - closed cup
Point d'éclair (°C)
113 °C - closed cup
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Zhiqian Wang et al.
Tetrahedron letters, 53(5), 477-479 (2012-05-01)
A novel MCAP-cycloaddition sequence has been applied to the facile synthesis of β-carboline intermediates to gain rapid access to novel derivatives of yohimbine-like and corynanthe-like compounds that may be easily diversified by cross-coupling reactions and N-derivatizations to generate small compound
Total synthesis of indoles from Tricholoma species via Bartoli/heteroaryl radical methodologies.
A Dobbs
The Journal of organic chemistry, 66(2), 638-641 (2001-06-30)
The structure of monobrominated ethyl indole-3-carboxylate and the preparation of 7-bromoindole.
Leggetter BE and Brown RK.
Canadian Journal of Chemistry, 38(9), 1467-1471 (1960)
Jin-Hyung Lee et al.
Applied microbiology and biotechnology, 97(10), 4543-4552 (2013-01-16)
Human pathogens can readily develop drug resistance due to the long-term use of antibiotics that mostly inhibit bacterial growth. Unlike antibiotics, antivirulence compounds diminish bacterial virulence without affecting cell viability and thus, may not lead to drug resistance. Staphylococcus aureus
J Y Kim et al.
Letters in applied microbiology, 41(2), 163-168 (2005-07-22)
To establish multicomponent phenol hydroxylases (mPHs) as novel biocatalysts for producing dyestuffs and hydroxyindoles such as 7-hydroxyindole (7-HI) from indole and its derivatives. We have isolated Pseudomonas sp. KL33, which possesses a phenol degradation pathway similar to that found in
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique