341673
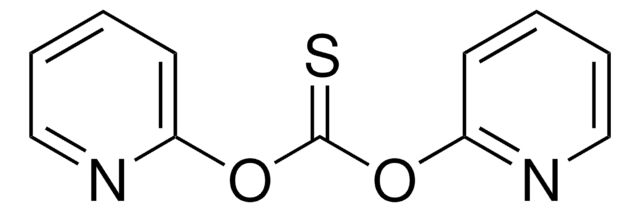
1,1′-Thiocarbonyldi-2(1H)-pyridone
97%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C11H8N2O2S
Numéro CAS:
Poids moléculaire :
232.26
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
97%
Pf
163-166 °C (lit.)
Groupe fonctionnel
thiourea
Chaîne SMILES
O=C1C=CC=CN1C(=S)N2C=CC=CC2=O
InChI
1S/C11H8N2O2S/c14-9-5-1-3-7-12(9)11(16)13-8-4-2-6-10(13)15/h1-8H
Clé InChI
KXMMNJQMGILZDB-UHFFFAOYSA-N
Application
1,1′-Thiocarbonyldi-2(1H)-pyridone was used in the preparation of:
- thio-analogs of thioureas
- sulforaphane
- 2-furan-2-yl-3-hydroxy-6-isothiocyanato-chromen-4-one
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Andrey S Klymchenko et al.
The journal of physical chemistry. B, 112(38), 12050-12055 (2008-09-05)
Herein, the efficient interaction of an environment-sensitive fluorophore that undergoes excited-state intramolecular proton transfer (ESIPT) with DNA has been realized by conjugation of a 3-hydroxychromone (3HC) with polycationic spermine. On binding to a double-stranded DNA (dsDNA), the ratio of the
C Clifford Conaway et al.
Cancer research, 65(18), 8548-8557 (2005-09-17)
We have shown previously that naturally occurring isothiocyanates derived from cruciferous vegetables and their N-acetylcysteine conjugates inhibit lung adenoma formation induced by tobacco carcinogens in A/J mice at the post-initiation stage. The tumor-inhibitory activity by these compounds is linked with
Sun-Young Jang et al.
Bioorganic & medicinal chemistry letters, 14(15), 3881-3883 (2004-07-01)
A series of 4-arylpiperazin-1-yl-3-phenyloxazolidin-2-one derivatives with diversification of the N-substituents such as methylene O-linked heterocycles, thioamide, dithiocarbamate, thiourea, and thiocarbamate were synthesized and evaluated as antibacterial agents. Their in vitro activities (MIC) were evaluated against MRSA and VRE resistant Gram-positive
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique