A6848
Aztreonam
analytical standard
Synonyme(s) :
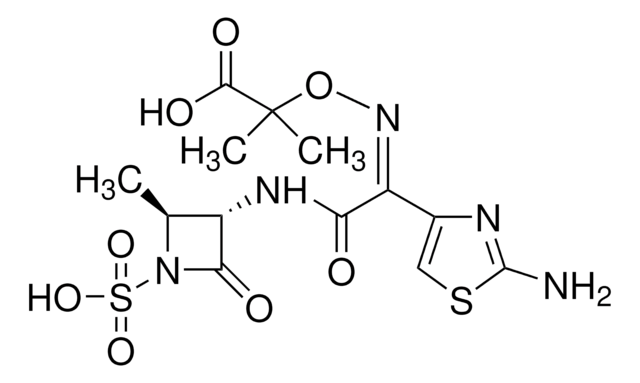
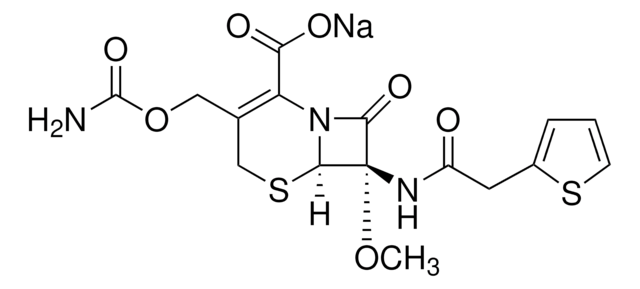
[2S-[2α,3β(Z)]]-2-[[[1-(2-Amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methylpropanoic acid
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Forme
powder or crystals
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
forensics and toxicology
pharmaceutical (small molecule)
veterinary
Chaîne SMILES
C[C@H]1[C@H](NC(=O)\C(=N/OC(C)(C)C(O)=O)c2csc(N)n2)C(=O)N1S(O)(=O)=O
InChI
1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1
Clé InChI
WZPBZJONDBGPKJ-VEHQQRBSSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Determination of aztreonam in two simulated lung fluid solutions― artificial lysosomal fluid and gamble solution by a UV spectroscopic method
- Multi-residue analysis of eight β-lactams by ultra-high performance liquid chromatography (UHPLC) combined with a photo-diode array (PDA) detector in human plasma & serum samples
- Simultaneous quantitative analysis of nine β-lactams by UHPLC along with tandem mass spectrometry (MS/MS) in human plasma samples
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique