O-024
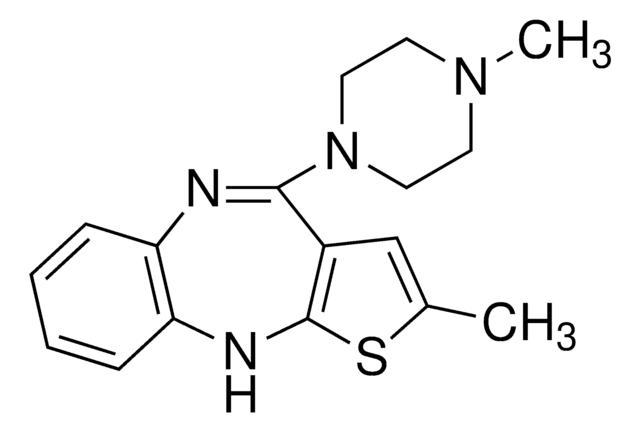
Olanzapine solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produits recommandés
Qualité
certified reference material
Forme
liquid
Caractéristiques
Snap-N-Spike®/Snap-N-Shoot®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
Concentration
1.0 mg/mL in acetonitrile
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
clinical testing
Format
single component solution
Température de stockage
−20°C
Chaîne SMILES
CN1CCN(CC1)C2=Nc3ccccc3Nc4sc(C)cc24
InChI
1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3
Clé InChI
KVWDHTXUZHCGIO-UHFFFAOYSA-N
Informations sur le gène
human ... DRD2(1813) , DRD3(1814) , DRD4(1815) , HTR2A(3356) , HTR2C(3358)
Description générale
Application
- Metabolic impact of Olanzapine in bipolar disorder: A network meta-analysis of randomized-controlled trials assessed the metabolic effects of antipsychotics and mood stabilizers, including Olanzapine, in patients with bipolar disorder, providing insights into its pharmacodynamic properties and implications for treatment optimization (Kong et al., 2024).
- Machine learning in schizophrenia research: Olanzapine′s effectiveness was evaluated through a systematic review employing machine learning to predict violent behaviors in schizophrenia spectrum disorders. This study underscores the relevance of antipsychotic treatment profiling in enhancing patient-specific therapeutic strategies (Delvecchio et al., 2024).
- Solubility and bioavailability enhancement: Research focused on the evaluation of polymeric matrices for loading poorly water-soluble drugs, like Olanzapine, into mesoporous silica. This study advances the development of more effective and bioavailable oral liquid formulations of Olanzapine (Barmpalexis et al., 2024).
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
35.6 °F - closed cup
Point d'éclair (°C)
2 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique