465194
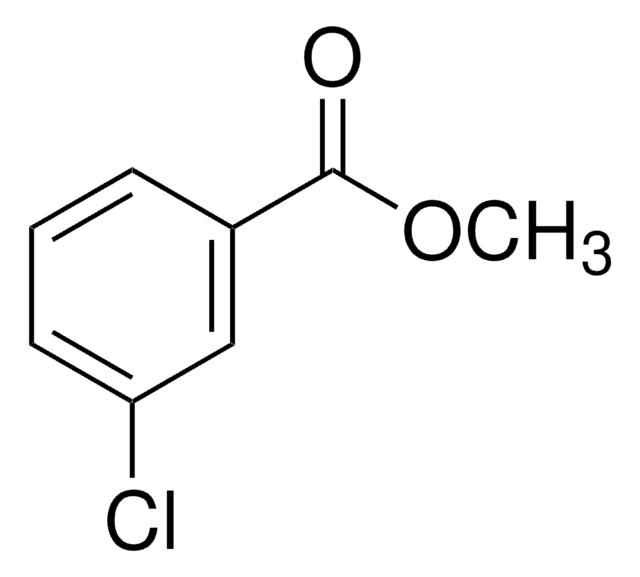
Methyl 2-chlorobenzoate
≥98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
ClC6H4CO2CH3
Numéro CAS:
Poids moléculaire :
170.59
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
≥98%
Forme
liquid
Indice de réfraction
n20/D 1.536 (lit.)
pb
86-88 °C/2.5 mmHg (lit.)
Densité
1.191 g/mL at 25 °C (lit.)
Chaîne SMILES
COC(=O)c1ccccc1Cl
InChI
1S/C8H7ClO2/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5H,1H3
Clé InChI
JAVRNIFMYIJXIE-UHFFFAOYSA-N
Description générale
Methyl 2-chlorobenzoate, a methyl 2-halobenzoate, is an ester. It can be synthesized from 2-chlorobenzoyl chloride. Its reduction with NaBH4 in diglyme at 162°C affords 2-chlorobenzyl alcohol.
Application
Methyl 2-chlorobenzoate may be used in the synthesis of various quinazolinone derivatives. It was used as starting reagent in the synthesis of 2-chlorobenzohydrazide.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
228.2 °F - closed cup
Point d'éclair (°C)
109.00 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Navin B Patel et al.
Scientia pharmaceutica, 78(2), 171-193 (2010-12-24)
In attempt to find new pharmacologically active molecules, we report here the synthesis and in vitro antimicrobial activity of various 3-(1,3,4-oxadiazol-2-yl)-quinazolin-4(3H)-ones. The antimicrobial activity of title compounds were examined against two gram positive bacteria (S. aureus, S. pyogenes), two gram
Reductions of Carboxylic Acids and Esters with NaBH4 in Diglyme at 162?C.
Zhu H-J and Pittman Jr CU.
Synthetic Communications, 33(10), 1733-1750 (2003)
Cheng Huang et al.
Chemical communications (Cambridge, England), (47)(47), 6333-6335 (2008-12-03)
We have developed a general and highly efficient copper-catalyzed method for synthesis of quinazoline and quinazolinone derivatives, the target products were obtained in good to excellent yields via cascade reactions of amidine hydrochlorides with substituted 2-halobenzaldehydes, 2-halophenylketones, or methyl 2-halobenzoates
Nicholas R Larson et al.
Journal of medical entomology, 57(1), 187-191 (2019-09-10)
Common bed bug Cimex lectularius (L.) (Hemiptera: Cimicidae) infestations are on the rise and due to the development of pesticide resistance they are becoming more difficult to control, affordably. We evaluated a naturally occurring compound methyl benzoate (MB) and related
Yan Feng et al.
Scientific reports, 8(1), 7902-7902 (2018-05-23)
Benzyl methyl ester, also known as methyl benzoate (MB), is a volatile organic compound that exists naturally as a floral fragrance in many plants. Our behavioral bioassays show that MB and some of its naturally occurring and synthetic analogs kill
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique