161160
α-Bromocinnamaldehyde
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
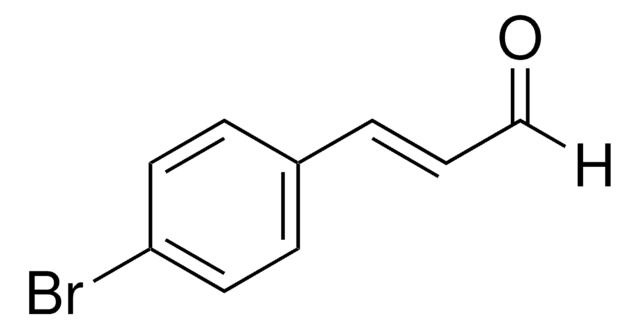
Formule linéaire :
C6H5CH=C(Br)CHO
Numéro CAS:
Poids moléculaire :
211.06
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Produits recommandés
Essai
98%
Forme
solid
Pf
66-68 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
Br\C(C=O)=C/c1ccccc1
InChI
1S/C9H7BrO/c10-9(7-11)6-8-4-2-1-3-5-8/h1-7H/b9-6-
Clé InChI
WQRWNOKNRHCLHV-TWGQIWQCSA-N
Description générale
α-Bromocinnamaldehyde is commonly employed as an anti-mildew agent in commercial products.
Application
α-Bromocinnamaldehyde was used in the synthesis of 3,4-diaryl 1H-pyrazoles. It was also used in the preparation of spiro imidazolidine-oxazolidine intermediate via guanidinium ylide mediated aziridination.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Ya Zhang et al.
International immunopharmacology, 14(1), 107-113 (2012-06-20)
Early experiments showed cinnamaldehyde had obvious therapeutic effect on viral myocarditis, but cinnamaldehyde was unstable in vivo. To overcome this limitation, we used cinnamaldehyde as a lead compound to synthesize α-bromo-4-chlorocinnamaldehyde (BCC). In the present study, we compared the therapeutic
S Kojima et al.
Eisei Shikenjo hokoku. Bulletin of National Institute of Hygienic Sciences, (107)(107), 21-25 (1989-01-01)
The amount of alpha-bromocinnamaldehyde (BCA), an anti-mildew agent, in some commercial products, was examined by high performance liquid chromatography (HPLC) using the following conditions: column, Nucleosil 50-5 (Nagel, 250 mm x 4.6 mm i.d.); mobile phase, hexane-chloroform (12:5); flow rate
Boris O A Tasch et al.
Organic & biomolecular chemistry, 11(36), 6113-6118 (2013-08-09)
The Masuda borylation-Suzuki coupling (MBSC) sequence was successfully extended to the challenging coupling of vinylhalides with various (hetero)arylhalides using sterically hindered phosphane ligands. Starting from (hetero)arylhalides and α-bromocinnamaldehyde, the sequentially Pd-catalyzed process selectively furnishes α,β-substituted cinnamaldehydes without affecting the reactivity
J Momma et al.
Eisei Shikenjo hokoku. Bulletin of National Institute of Hygienic Sciences, (107)(107), 29-36 (1989-01-01)
Bromocinnamic aldehyde (BCA), an antibacterial/antifungal agent, was tested for its chronic toxicity/carcinogenicity in female Slc:ddY mice. The animals received 0.25%, 1.0% and 4.0% of BCA dissolved in olive oil applied to the shaved back skin area twice a week for
Wannaporn Disadee et al.
The Journal of organic chemistry, 71(17), 6600-6603 (2006-08-12)
We successfully isolated a spiro imidazolidine-oxazolidine intermediate in the reaction of guanidinium ylide mediated aziridination using alpha-bromocinnamaldehyde. X-ray crystallographic analysis unambiguously revealed that the stereogenic centers of the spiro intermediate were in a trans configuration. The role of the spiro
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique



![[1,1′-Bis(diphénylphosphino)ferrocène]dichloropalladium(II), complexe avec le dichlorométhane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)




