142670
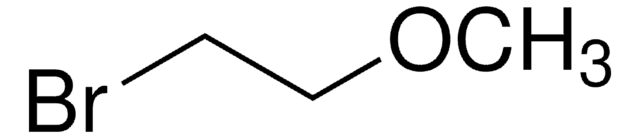
Chloromethyl ethyl ether
95%
Synonym(s):
(Chloromethoxy)ethane, Ethoxychloromethane, Ethoxymethyl chloride, Ethyl chloromethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OCH2Cl
CAS Number:
Molecular Weight:
94.54
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.404 (lit.)
bp
82 °C (lit.)
density
1.019 g/mL at 25 °C (lit.)
functional group
chloro
ether
storage temp.
2-8°C
SMILES string
CCOCCl
InChI
1S/C3H7ClO/c1-2-5-3-4/h2-3H2,1H3
InChI key
FCYRSDMGOLYDHL-UHFFFAOYSA-N
Application
Chloromethyl ethyl ether can be used to prepare:
- 6-Benzyl-1-(ethoxymethyl)-5-iodopyrimidine-2,4(1H,3H)-dione, a key intermediate in the preparation of MKC-442 analog.
- Acylation catalyst for alcohols named 1,3-bis-[(R)-1-(2-naphthyl)ethyl]imidazoliumchloride by reacting with glyoxal-bis-[(R)-1-(2-naphthyl)ethyl]imine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
66.2 °F - closed cup
Flash Point(C)
19 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
bis(Chloromethyl) ether and technical-grade chloromethyl methyl ether.
Report on carcinogens : carcinogen profiles, 10, 56-57 (2004-08-24)
Chiral N-heterocyclic carbenes as asymmetric acylation catalysts
Suzuki Y, et al.
Tetrahedron, 62(2-3), 302-310 (2006)
Three Routes for the Synthesis of 6-Benzyl-1-ethoxymethyl-2, 4-dioxo-1, 2, 3, 4-tetrahydropyrimidine-5-carbaldehyde
Petersen L, et al.
Synthesis, 2001(04), 0559-0564 (2001)
K Takahashi et al.
Mutation research, 156(3), 153-161 (1985-06-01)
The mutagenic characteristics of formaldehyde on bacteria were examined. All the tester strains of Escherichia coli deficient in DNA-repair enzymes tested in the present study were significantly more sensitive to the killing effect of formaldehyde than the corresponding wild-type strain.
Tadeusz J Szalaty et al.
International journal of biological macromolecules, 119, 431-437 (2018-07-29)
In this research we use ionic liquids in combination with mild process conditions to provide a selective increase in the content of carbonyl groups in the kraft lignin structure. Such modification can improve the properties of the pristine biopolymer. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service