T7191
Paclitaxel
from semisynthetic, ≥98%
About This Item
Productos recomendados
origen biológico
semisynthetic
Análisis
≥98%
impurezas
natural taxane impurities, none detected (except ≤0.5% paclitaxel degradation products.)
mp
213 °C (dec.) (lit.)
solubilidad
DMSO: soluble 50 mg/mL
methanol: soluble 50 mg/mL, clear, colorless
H2O: soluble (hydrolyzes)
ethanol: soluble
espectro de actividad antibiótica
neoplastics
Modo de acción
DNA synthesis | interferes
emisor
Bristol-Myers Squibb
temp. de almacenamiento
−20°C
cadena SMILES
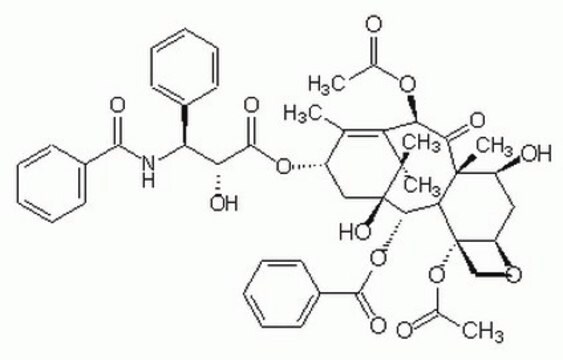
[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)c6ccccc6)c7ccccc7
InChI
1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
Clave InChI
RCINICONZNJXQF-MZXODVADSA-N
Información sobre el gen
human ... BCL2(596) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Características y beneficios
Precaución
Nota de preparación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Muta. 2 - Repr. 1B - STOT RE 1
Órganos de actuación
Central nervous system,Bone marrow,Cardio-vascular system
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Discover Bioactive Small Molecules for ADME/Tox
Contenido relacionado
Explore protein pathway analysis including chemical library screening and modulating pathways with small molecules.
El estudio de las vías proteicas es una parte fundamental de la investigación en el descubrimiento de fármacos y el desarrollo de medicamentos. Explore más sobre el análisis de las vías proteicas, incluidos el cribado de quimiotecas, la investigación de la actividad de proteínas y enzimas, y la modulación de las vías proteicas mediante la utilización de moléculas pequeñas.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico