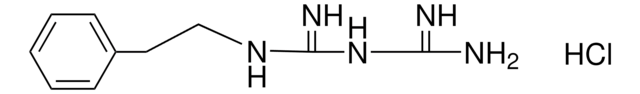
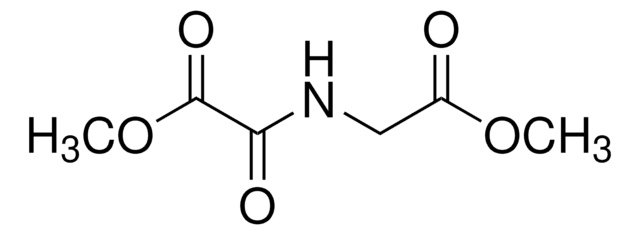
T5580
Myxothiazol
from Myxococcus fulvus Mx f85, ≥98% (HPLC), lyophilized solid, antibiotic
Sinónimos:
Myxothiazol A
About This Item
Productos recomendados
product name
Myxothiazol, from Myxococcus fulvus Mx f85, ≥98% (HPLC)
origen biológico
Myxococcus fulvus Mx f85
Nivel de calidad
Análisis
≥98% (HPLC)
condiciones de almacenamiento
protect from light
under inert gas
solubilidad
chloroform: soluble 9.80-10.20 mg/mL, clear, colorless to yellow
DMSO: soluble
acetone: soluble
dichloromethane: soluble
ethanol: soluble
ethyl acetate: soluble
methanol: soluble
espectro de actividad antibiótica
fungi
Modo de acción
enzyme | inhibits
temp. de almacenamiento
−20°C
cadena SMILES
CO[C@@H](\C=C\c1csc(n1)-c2csc(n2)[C@@H](C)\C=C\C=C\C(C)C)[C@@H](C)\C(OC)=C\C(N)=O
InChI
1S/C25H33N3O3S2/c1-16(2)9-7-8-10-17(3)24-28-20(15-33-24)25-27-19(14-32-25)11-12-21(30-5)18(4)22(31-6)13-23(26)29/h7-18,21H,1-6H3,(H2,26,29)/b9-7+,10-8+,12-11+,22-13-/t17-,18+,21-/m0/s1
Clave InChI
XKTFQMCPGMTBMD-ZDBABOMLSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Acciones bioquímicas o fisiológicas
Nota de preparación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 2 Oral
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico