SML0287
680C91
≥98% (HPLC)
Sinónimos:
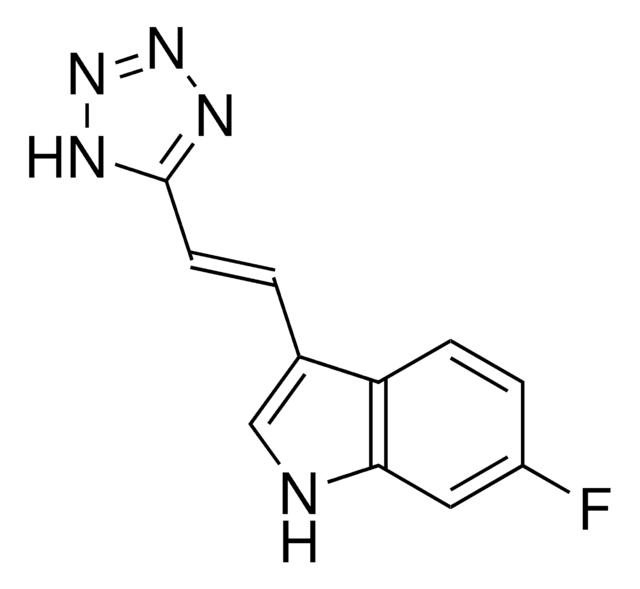
6-Fluoro-3-[(1E)-2-(3-pyridinyl)ethenyl)-1H-indole
About This Item
Productos recomendados
Nivel de calidad
Análisis
≥98% (HPLC)
formulario
powder
color
white to beige
solubilidad
DMSO: ≥10 mg/mL
temp. de almacenamiento
2-8°C
cadena SMILES
Fc1ccc2c(\C=C\c3cccnc3)c[nH]c2c1
InChI
1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+
Clave InChI
YBSDQTBCNYWBMX-ONEGZZNKSA-N
Aplicación
- as a tryptophan 2,3 dioxygenase (TDO) inhibitor to study its effects on the pigmentation in Doryteuthis pealeii embryos
- as a TDO inhibitor to study its effects on esophageal squamous cell carcinoma in xenograft tumor assay
- as a tryptophan 2,3 dioxygenase 2 (TDO2) inhibitor to study its effects on toxic fragment formation in human embryonic kidney cells
Acciones bioquímicas o fisiológicas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Dam. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![2,4-Dichlorothieno[3,2-d]pyrimidine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/408/704/0886c958-6ca7-40e3-91c0-ecc81fbfe483/640/0886c958-6ca7-40e3-91c0-ecc81fbfe483.png)