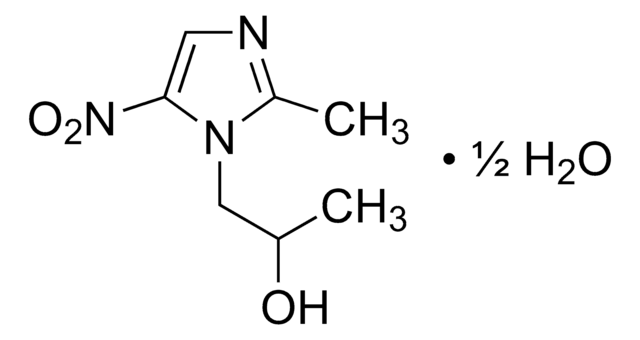
PHR1293
Nimodipine
Pharmaceutical Secondary Standard; Certified Reference Material
Sinónimos:
Nimodipine, 1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 2-methoxyethyl 1-methylethyl ester, Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
About This Item
Productos recomendados
grado
certified reference material
pharmaceutical secondary standard
Nivel de calidad
Agency
traceable to BP 651
traceable to Ph. Eur. N0850000
traceable to USP 1463858
familia API
nimodipine
CofA
current certificate can be downloaded
técnicas
HPLC: suitable
gas chromatography (GC): suitable
aplicaciones
pharmaceutical (small molecule)
formato
neat
temp. de almacenamiento
2-8°C
cadena SMILES
COCCOC(=O)C1=C(C)NC(C)=C(C1c2cccc(c2)[N+]([O-])=O)C(=O)OC(C)C
InChI
1S/C21H26N2O7/c1-12(2)30-21(25)18-14(4)22-13(3)17(20(24)29-10-9-28-5)19(18)15-7-6-8-16(11-15)23(26)27/h6-8,11-12,19,22H,9-10H2,1-5H3
Clave InChI
UIAGMCDKSXEBJQ-UHFFFAOYSA-N
Información sobre el gen
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779) , NR3C2(4306)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Aplicación
Nota de análisis
Otras notas
Nota al pie de página
Producto relacionado
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Choose from one of the most recent versions:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico