42612
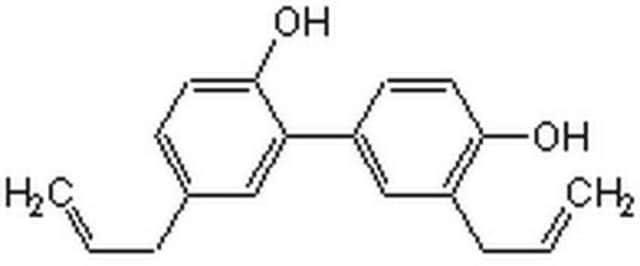
Honokiol
analytical standard
Sinónimos:
5,3′-Diallyl-2,4′-dihydroxybiphenyl, NSC 293100
About This Item
Productos recomendados
grado
analytical standard
Nivel de calidad
Análisis
≥98.0% (HPLC)
caducidad
limited shelf life, expiry date on the label
técnicas
HPLC: suitable
gas chromatography (GC): suitable
aplicaciones
food and beverages
formato
neat
temp. de almacenamiento
2-8°C
cadena SMILES
Oc1ccc(cc1CC=C)-c2cc(CC=C)ccc2O
InChI
1S/C18H18O2/c1-3-5-13-7-9-18(20)16(11-13)14-8-10-17(19)15(12-14)6-4-2/h3-4,7-12,19-20H,1-2,5-6H2
Clave InChI
FVYXIJYOAGAUQK-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
Acciones bioquímicas o fisiológicas
Productos recomendados
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Aquatic Chronic 2 - Eye Dam. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Choose from one of the most recent versions:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico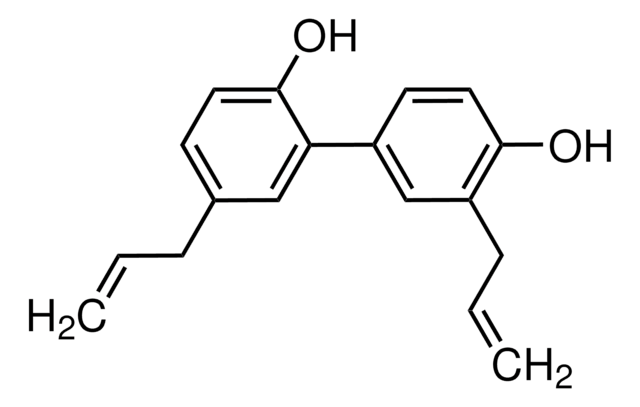

![Dibenzo[a,h]pyrene BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/246/746/f3144b1e-aff5-4c36-8701-769da2ffe8c0/640/f3144b1e-aff5-4c36-8701-769da2ffe8c0.png)





