G10806
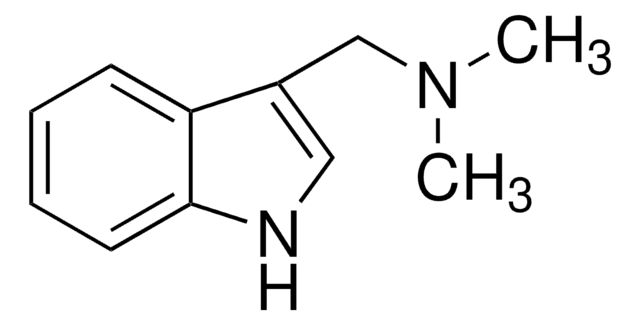
Gramine
97.5%
Sinónimos:
3-(Dimethylaminomethyl)indole, Donaxine, NSC 16892
About This Item
Productos recomendados
Análisis
97.5%
mp
132-134 °C (lit.)
cadena SMILES
CN(C)Cc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-13(2)8-9-7-12-11-6-4-3-5-10(9)11/h3-7,12H,8H2,1-2H3
Clave InChI
OCDGBSUVYYVKQZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- Dopamine D2 receptor antagonists
- Anti-malarial drugs
- 5-indolyl-Mannich bases
- Proliferation inhibitors
- Inhibitors of human mast cell chymase
- Preparation of DL-tryptophan
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- 3-vinylindoles
- Serotonin 5-HT6 receptor ligand templates
- Selective protein kinase c delta (PKCδ) down regulators
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
332.6 °F
Punto de inflamabilidad (°C)
167 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico