930520
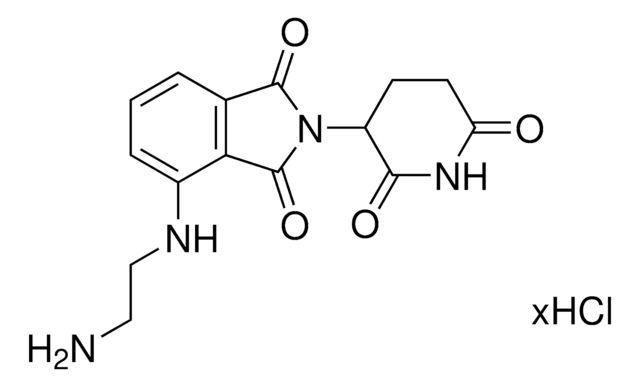
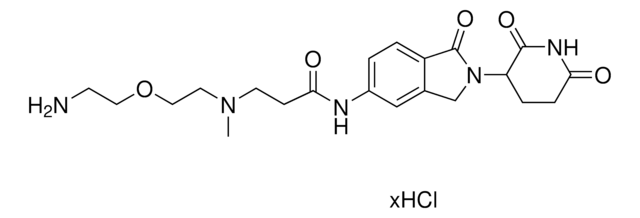
Pomalidomide-PEG2-C2-NH2 hydrochloride
≥95%
Sinónimos:
1H-Isoindole-1,3(2H)-dione, 4-[[2-[2-(2-aminoethoxy)ethoxy]ethyl]amino]-2-(2,6-dioxo-3-piperidinyl), 4-[[2-[2-(2-Aminoethoxy)ethoxy]ethyl]amino]-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
About This Item
Productos recomendados
ligand
pomalidomide
Nivel de calidad
Análisis
≥95%
formulario
powder
grupo funcional
amine
temp. de almacenamiento
2-8°C
cadena SMILES
O=C1NC(C(CC1)N2C(C3=C(C2=O)C(NCCOCCOCCN)=CC=C3)=O)=O
Categorías relacionadas
Aplicación
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Otras notas
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico