917699
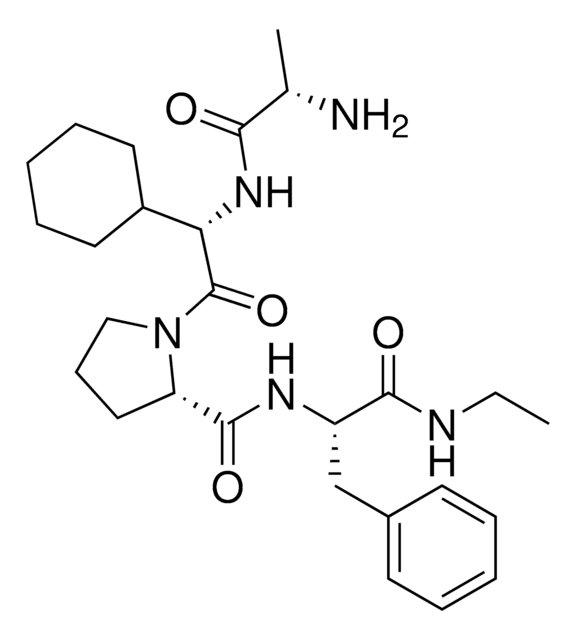
BocA1V2PF2-NHC10-NH2
≥85%
Sinónimos:
tert-Butyl ((S)-1-(((S)-2-((S)-2-(((S)-1-((10-Aminodecyl)amino)-3-(4-fluorophenyl)-1-oxopropan-2-yl)carbamoyl)pyrrolidin-1-yl)-1-cyclohexyl-2-oxoethyl)amino)-1-oxopropan-2-yl)carbamate, AVP conjugate for IAP-mediated protein degrader development, SNIPER building block
Seleccione un Tamaño
381,00 €
Seleccione un Tamaño
About This Item
381,00 €
Productos recomendados
ligand
BocA1V2PF2
Nivel de calidad
Ensayo
≥85%
Formulario
powder
idoneidad de la reacción
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
grupo funcional
amine
temp. de almacenamiento
2-8°C
cadena SMILES
C[C@H](NC(OC(C)(C)C)=O)C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(NCCCCCCCCCCN)=O)CC2=CC=C(C=C2)F)=O)=O)C3CCCCC3)=O
InChI
1S/C40H65FN6O6/c1-28(44-39(52)53-40(2,3)4)35(48)46-34(30-17-12-11-13-18-30)38(51)47-26-16-19-33(47)37(50)45-32(27-29-20-22-31(41)23-21-29)36(49)43-25-15-10-8-6-5-7-9-14-24-42/h20-23,28,30,32-34H,5-19,24-27,42H2,1-4H3,(H,43,49)(H,44,52)(H,45,50)(H,46,48)/t
Clave InChI
AFZPWVXOGIXQBS-MTZHBSBLSA-N
Aplicación
Building blocks in this series:
917974 BocA1V2PF2
917443 BocA1V2PF2-NHC6-NH2
917699 BocA1V2PF2-NHC10-NH2
916951 BocA1V2PF1-NHPEG1-NH2
917214 BocA1V2PF1-NHPEG3-NH2
Technology Spotlight: Degrader Building Blocks with Inhibitor of Apoptosis Protein (IAP) In Silico-Derived Ligands
Otras notas
Información legal
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Targeted protein degradation reduces disease-relevant proteins in cells using small molecules, hijacking endogenous proteolysis systems.
Plate of 80 ligands against E3 ligase IAP designed by ComInnex; allows creation of bifunctional targeted protein degraders or molecular glues.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Filtros activos
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico