803243
Sulfo-SMPB (sulfosuccinimidyl 4-(N-maleimidophenyl)butyrate)
About This Item
Productos recomendados
formulario
powder
Nivel de calidad
mol peso
458.38
idoneidad de la reacción
reagent type: cross-linking reagent
condiciones de almacenamiento
desiccated
solubilidad
water: soluble
grupo funcional
maleimide
Condiciones de envío
ambient
temp. de almacenamiento
−20°C
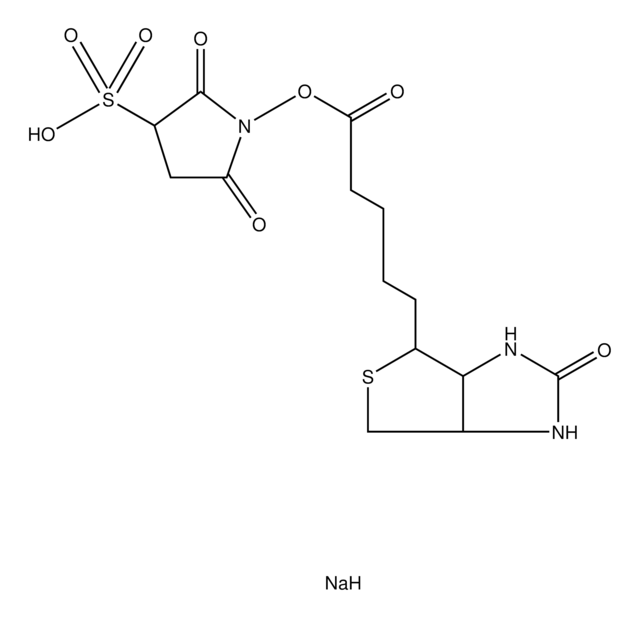
cadena SMILES
O=C1CC(S(=O)([O-])=O)C(N1OC(CCCC2=CC=C(N3C(C=CC3=O)=O)C=C2)=O)=O.[Na+]
InChI
1S/C18H16N2O9S.Na/c21-14-8-9-15(22)19(14)12-6-4-11(5-7-12)2-1-3-17(24)29-20-16(23)10-13(18(20)25)30(26,27)28;/h4-9,13H,1-3,10H2,(H,26,27,28);/q;+1/p-1
Clave InChI
LSZBIKKARBHBPA-UHFFFAOYSA-M
Descripción general
Características y beneficios
- Reactive groups: sulfo-NHS ester and maleimide
- Reactive towards: amino and sulfhydryl groups
- Extended chain length limits steric hindrance
- Non-cleavable
- Water-soluble (compare to SMPB)
- Membrane impermeable, allowing for cell surface labeling
Precaución
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico