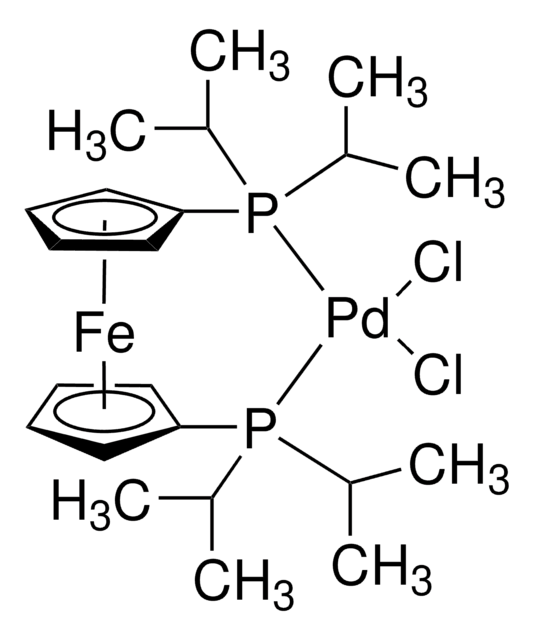
678740
(AMPHOS)2PdCl2
Sinónimos:
Bis[4-[Bis(tert-butyl)phosphino]-N, N-Dimethylbenzenamide]dichloropalladium
About This Item
Productos recomendados
formulario
solid
Nivel de calidad
idoneidad de la reacción
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
grupo funcional
phosphine
cadena SMILES
Cl[Pd]Cl.CN(c1ccc(P(C(C)(C)C)C(C)(C)C)cc1)C.CN(c2ccc(P(C(C)(C)C)C(C)(C)C)cc2)C
InChI
1S/2C16H28NP.2ClH.Pd/c2*1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;;;/h2*9-12H,1-8H3;2*1H;/q;;;;+2/p-2
Clave InChI
DWOZNANUEDYIOF-UHFFFAOYSA-L
Descripción general
For small scale and high throughput uses, product is also available as ChemBeads (927791)
Aplicación
- In the enantioselective construction of indole-fused bicyclo[3.2.1]-octanes via an aminopalladition-triggered Heck-type reaction.
- In the synthesis of phenanthridine derivatives from ortho bromo N-tosylhydrazones and 2-aminophenylboronic ester via Suzuki cross-coupling reaction followed by intramolecular condensation reaction.
- In the synthesis of indenones by Pd-catalyzed annulation of an ortho-halobenzyl alcohol with internal alkynes.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Ligand used to prepare a palladium dichloride catalyst on treatment with PdCl2(COD). The catalyst effectively cross-couples aryl boronic acids with heteroaryl chlorides.
A variety of palladium-catalyzed cross-coupling reactions can be run under room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands.
Protocolos
TPGS-750-M surfactant enables various reactions in water at room temperature, enhancing efficiency and versatility in synthesis.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)








![[1,1′-Bis(di-cyclohexylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/136/854/a3142b2e-900c-47e5-8100-e48add9f4db6/640/a3142b2e-900c-47e5-8100-e48add9f4db6.png)