367710
Trichloroacetic anhydride
technical grade, 95%
Sinónimos:
(2,2,2-Trichloroacetyl) 2,2,2-trichloroacetate, Trichloroacetic acid anhydride
About This Item
Productos recomendados
grado
technical grade
Análisis
95%
formulario
liquid
índice de refracción
n20/D 1.484 (lit.)
bp
139-141 °C/60 mmHg (lit.)
densidad
1.69 g/mL at 25 °C (lit.)
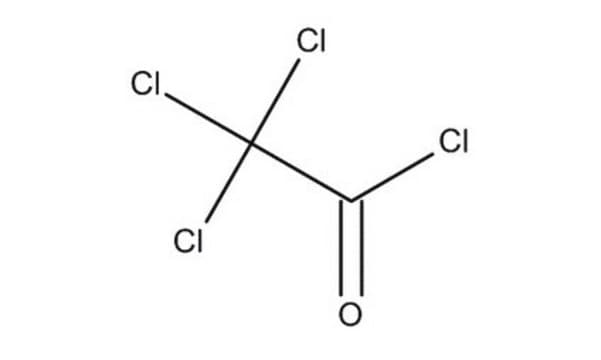
cadena SMILES
ClC(Cl)(Cl)C(=O)OC(=O)C(Cl)(Cl)Cl
InChI
1S/C4Cl6O3/c5-3(6,7)1(11)13-2(12)4(8,9)10
Clave InChI
MEFKFJOEVLUFAY-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
- Simultaneous analysis of amphetamine, methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA or Ecstasy) in urine samples by solid-phase extraction, derivatization and gas chromatography/mass spectrometry.
- Analysis of halogenated amphetamines in brain tissue from rats by gas chromatography with electron-capture detection (GC-ECD).
- Quantitative determination of the metabolites of l-alpha-acetylmethadol (LAAM), such as noracetylmethadol, dinoracetylmethadol, methadol and normethadol by electron capture gas-liquid chromatography.
Palabra de señalización
Danger
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Oral - Skin Corr. 1A
Código de clase de almacenamiento
8A - Combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico