Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
156329
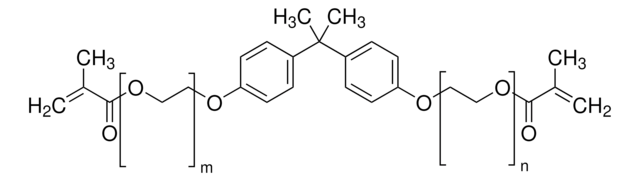
Bisphenol A dimethacrylate
>98%
Sinónimos:
2,2-Bis(4-hydroxyphenyl)propane dimethacrylate, 2,2-Bis(4-methacryloxyphenyl)propane, 2,2-Bis(4-methacryloyloxyphenyl)propane, 4,4′-Isopropylidenediphenol dimethacrylate, BPADMA
Seleccione un Tamaño
69,20 €
Seleccione un Tamaño
About This Item
69,20 €
Productos recomendados
Nivel de calidad
Ensayo
>98%
Formulario
solid
mp
72-74 °C (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
CC(=C)C(=O)Oc1ccc(cc1)C(C)(C)c2ccc(OC(=O)C(C)=C)cc2
InChI
1S/C23H24O4/c1-15(2)21(24)26-19-11-7-17(8-12-19)23(5,6)18-9-13-20(14-10-18)27-22(25)16(3)4/h7-14H,1,3H2,2,4-6H3
Clave InChI
QUZSUMLPWDHKCJ-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
Aplicación
- A template in the synthesis of molecularly imprinted polymers (MIPs). The use of BPA-DMA allows for the formation of specific cavities in the polymer matrix that match the shape and functional groups of BPA, enhancing the selectivity and affinity of the resulting polymer for its target analyte.
- A co-monomer in the synthesis of photopolymerized monoliths for capillary electrochromatography.
- A key component in the production of bisphenol A-glycidyl methacrylate (Bis-GMA), which is widely used in dental restorative materials due to its mechanical properties and potential for additional functionalities like antibacterial activity.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 respuesta-
¿Le ha resultado útil?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 respuesta-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf¿Le ha resultado útil?
-
Filtros activos
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico