130001
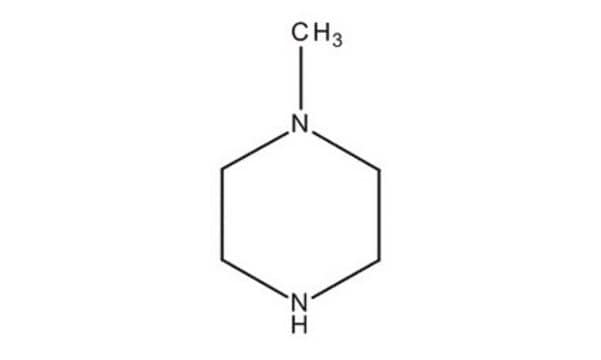
1-Methylpiperazine
99%
Sinónimos:
N-Methylpiperazine
About This Item
Productos recomendados
densidad de vapor
3.5 (vs air)
Nivel de calidad
Análisis
99%
formulario
liquid
índice de refracción
n20/D 1.466 (lit.)
bp
138 °C (lit.)
densidad
0.903 g/mL at 25 °C (lit.)
cadena SMILES
CN1CCNCC1
InChI
1S/C5H12N2/c1-7-4-2-6-3-5-7/h6H,2-5H2,1H3
Clave InChI
PVOAHINGSUIXLS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - Skin Sens. 1B
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
88.7 °F - closed cup
Punto de inflamabilidad (°C)
31.5 °C - closed cup
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico