B22984
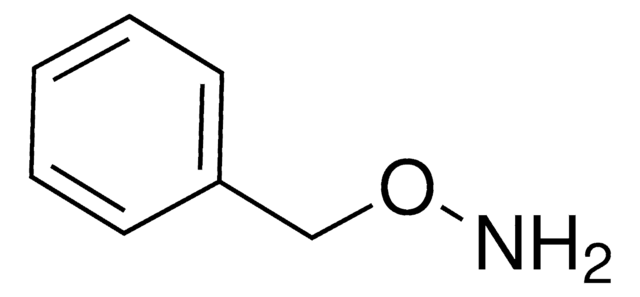
O-Benzylhydroxylamine hydrochloride
99%
Synonym(s):
Benzyloxyamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2ONH2 · HCl
CAS Number:
Molecular Weight:
159.61
Beilstein:
3687991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
mp
238 °C (subl.) (lit.)
SMILES string
Cl.NOCc1ccccc1
InChI
1S/C7H9NO.ClH/c8-9-6-7-4-2-1-3-5-7;/h1-5H,6,8H2;1H
InChI key
HYDZPXNVHXJHBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Effective reagent used to prepare α-hydroxybenzylamines from α-hydroxyketones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Stampf et al.
Die Pharmazie, 35(1), 43-44 (1980-01-01)
The study of the blood levels and tissue concentrations in mice to which 14C-benzyloxyamine hydrochloride was applied in the form of a spray and of a suspensoid aerosol evidenced the good abosrption of this pharmacon. Maximum blood levels were observed
Yin Luo et al.
ChemMedChem, 7(9), 1587-1593 (2012-07-20)
Forty-three oxime derivatives were synthesized by allowing O-benzylhydroxylamines to react with primary benzaldehydes or salicylaldehydes; these products were gauged as potential inhibitors of β-ketoacyl-(acyl-carrier-protein) synthase III (FabH). Among the 43 compounds, 38 are reported herein for the first time. These
G Cardillo et al.
Organic letters, 3(8), 1165-1167 (2001-05-12)
[reaction: see text]. The 1,4-addition of O-benzylhydroxylamine to alpha,beta-unsaturated imide 1 in the presence of BF3.Et2O proceeds with the preferential attack of the nucleophile on the Cbeta-re face. To explain this unexpected reactivity 1H, 13C, and 11B NMR investigations have
D F Magin
Journal of chromatography, 202(2), 255-261 (1980-12-19)
A qualitative and semi-quantitative method was established for the investigation of low-molecular-weight volatile carbonyl compounds in cigarette whole smoke. The carbonyls were trapped on a silica gel "column" and eluted with water. The aqueous solution was then treated with benzyloxyamine
Tetrahedron Letters, 32, 711-711 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service