All Photos(1)
About This Item
Empirical Formula (Hill Notation):
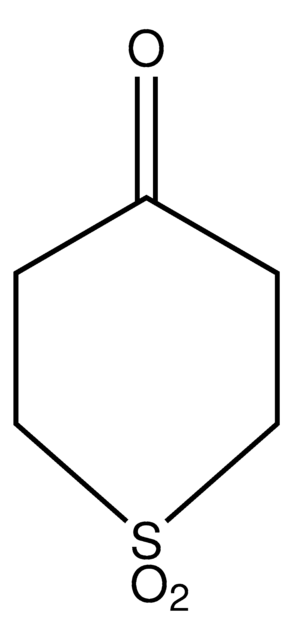
C5H8OS
CAS Number:
Molecular Weight:
116.18
Beilstein:
106464
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
60-64 °C (lit.)
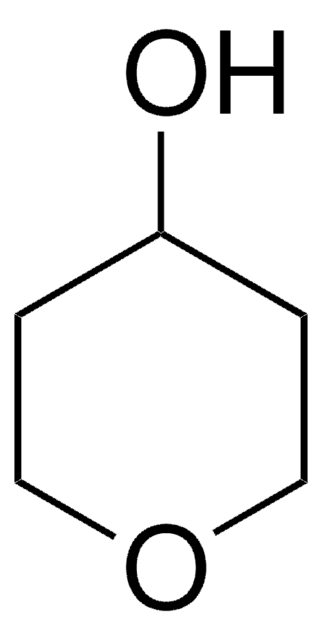
SMILES string
O=C1CCSCC1
InChI
1S/C5H8OS/c6-5-1-3-7-4-2-5/h1-4H2
InChI key
OVRJVKCZJCNSOW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The diastereoselectivity of the aldol reaction of tetrahydro-4H-thiopyran-4-one has been studied.
Application
Tetrahydro-4H-thiopyran-4-one was used in the preparation of meso 1,9-diketones.
The product has been utilized in various condensation reactions for the preparation of dipeptides, spiroimidazolones, and tetrahydrocarbazoles and α-hydroxy esters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synlett, 1605-1605 (2007)
Journal of Heterocyclic Chemistry, 30, 81-81 (1993)
Dale E Ward et al.
Organic letters, 8(12), 2631-2634 (2006-06-02)
Meso 1,9-diketones (six to seven stereocenters) are readily obtained by stepwise or simultaneous two-directional aldol reactions of tetrahydro-4H-thiopyran-4-one with a thiopyran-derived aldehyde or dialdehyde. Enantioselective enolizations of these diketones with the lithium amide from (R,R)-bis(1-phenylethyl)amine occur with simultaneous kinetic resolution
Dale E Ward et al.
The Journal of organic chemistry, 67(5), 1618-1629 (2002-03-02)
The diastereoselectivity of the aldol reaction of tetrahydro-4H-thiopyran-4-one (3) with 1,4-dioxa-8-thiaspiro[4.5]decane-6-carboxaldehyde (9a) under a variety of conditions is examined. Under optimized conditions, three of the four possible diastereomers from this aldol reaction can be obtained selectively (3-16:1). Reactions of 9a
Synthesis, 672-672 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service