W421001
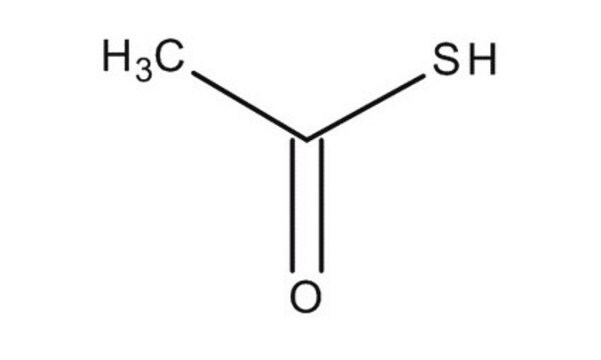
Thioacetic acid
96%
Sinónimos:
Thiacetic acid
About This Item
Productos recomendados
biological source
synthetic
Quality Level
assay
96%
refractive index
n20/D 1.465 (lit.)
bp
88-91.5 °C (lit.)
density
1.065 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
meaty; roasted
SMILES string
CC(S)=O
InChI
1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4)
InChI key
DUYAAUVXQSMXQP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Disclaimer
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
64.4 °F - closed cup
flash_point_c
18 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico