900948
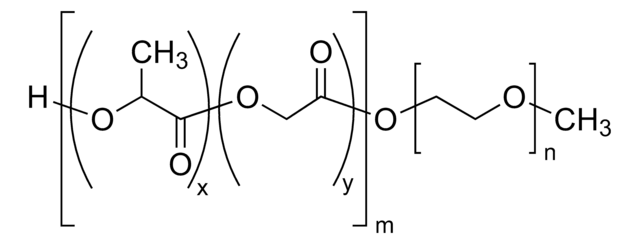
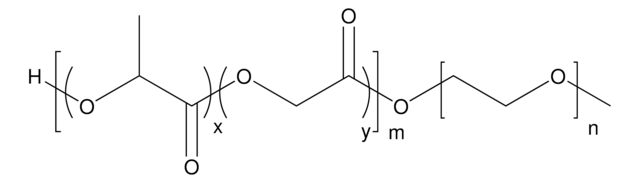
Poly(ethylene glycol) methyl ether-block-poly(lactide-co-glycolide)
PEG average Mn 5,000, PLGA Mn 15,000, lactide:glycolide 50:50
Sinónimos:
PEG-PLGA, Polyethylene glycol, mPEG-b-PLGA
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
H[(C3H4O2)x(C2H2O2)y]mO[C2H4O]nCH3
UNSPSC Code:
51171641
NACRES:
NA.23
Productos recomendados
form
crystals
Quality Level
feed ratio
lactide:glycolide 50:50
mol wt
PEG average Mn 5,000
PLGA Mn 15,000
impurities
≤5000 ppm (GC)
storage temp.
−20°C
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Contains ≤500 ppm impurities by GC, including trace monomer and residual organics.
Application
Biocompatible block copolymer. Can be used in the formation of nanoparticles for drug delivery. Potenial use in the targeted and/or controlled release of cancer drugs, anti-inflammatory drugs, antibiotics, or anesthetic agents.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
>230.9 °F
flash_point_c
> 110.5 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Fabienne Danhier et al.
Journal of controlled release : official journal of the Controlled Release Society, 133(1), 11-17 (2008-10-28)
The purpose of this study was to develop Cremophor EL-free nanoparticles loaded with Paclitaxel (PTX), intended to be intravenously administered, able to improve the therapeutic index of the drug and devoid of the adverse effects of Cremophor EL. PTX-loaded PEGylated
Yihan Xu et al.
Journal of biomedical materials research. Part B, Applied biomaterials, 105(6), 1692-1716 (2016-04-22)
Poly (lactic-co-glycolic acid) (PLGA) copolymers have been broadly used in controlled drug release applications. Because these polymers are biodegradable, they provide an attractive option for drug delivery vehicles. There are a variety of material, processing, and physiological factors that impact
R Gref et al.
Science (New York, N.Y.), 263(5153), 1600-1603 (1994-03-18)
Injectable nanoparticulate carriers have important potential applications such as site-specific drug delivery or medical imaging. Conventional carriers, however, cannot generally be used because they are eliminated by the reticulo-endothelial system within seconds or minutes after intravenous injection. To address these
Miles A Miller et al.
Nature communications, 6, 8692-8692 (2015-10-28)
Therapeutic nanoparticles (TNPs) aim to deliver drugs more safely and effectively to cancers, yet clinical results have been unpredictable owing to limited in vivo understanding. Here we use single-cell imaging of intratumoral TNP pharmacokinetics and pharmacodynamics to better comprehend their
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico