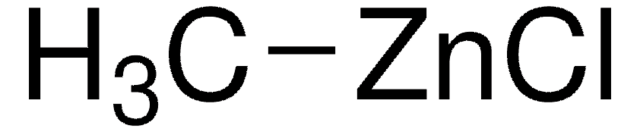417246
Dimethylzinc solution
1.0 M in heptane
Sinónimos:
Dimethylzinc
About This Item
Productos recomendados
form
liquid
Quality Level
concentration
1.0 M in heptane
bp
44-46 °C
density
0.724 g/mL at 25 °C
SMILES string
C[Zn]C
InChI
1S/2CH3.Zn/h2*1H3;
InChI key
AXAZMDOAUQTMOW-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Dimethylzinc is a diorganozinc reagent and nucleophile used in the synthesis of propargylic amines.
Application
- A catalyst with nickel for the stereoselective C−2 alkenylation and dialkenylation of pyridine derivatives by alkynes to give monoalkenylation products.
- A reagent with aldehydes and 2-methoxyaniline for the synthesis of enantioselective alkyl and aralkyl secondary amines via one-pot three-component coupling reaction in the presence of zirconium tetraisopropoxide.
- A methylating reagent for methylation of fluoroalkylated pyruvates in the presence of copper/chiral diphosphine catalyst.
signalword
Danger
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 2
target_organs
Central nervous system
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 3
flash_point_f
30.2 °F - closed cup
flash_point_c
-1 °C - closed cup
ppe
Faceshields, Gloves, Goggles
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico