357774
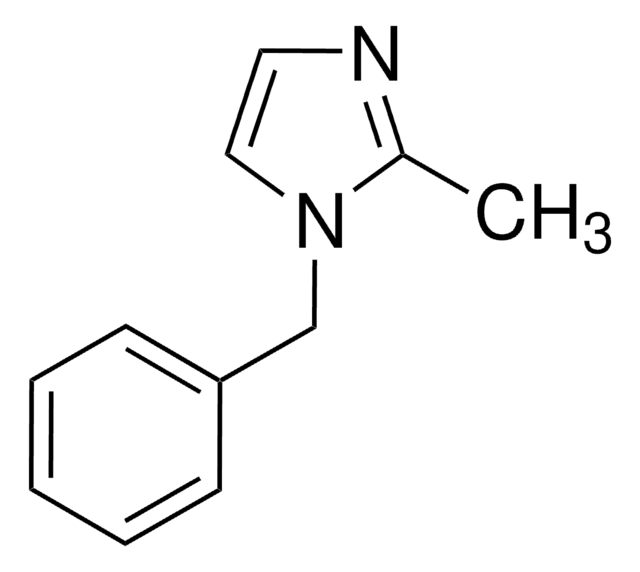
1-Phenylimidazole
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H8N2
Número de CAS:
Peso molecular:
144.17
Número MDL:
Código UNSPSC:
12352005
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
97%
Formulario
liquid
bp
142 °C/15 mmHg (lit.)
mp
13 °C (lit.)
densidad
1.14 g/mL at 25 °C (lit.)
cadena SMILES
c1ccc(cc1)-n2ccnc2
InChI
1S/C9H8N2/c1-2-4-9(5-3-1)11-7-6-10-8-11/h1-8H
Clave InChI
SEULWJSKCVACTH-UHFFFAOYSA-N
Descripción general
1-Phenylimidazole is an imidazole derivative. It induces 7-ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout (Oncorhynchus mykiss) hepatocytes. The S(1)→S(0) transition of 1-phenylimidazole has been investigated in a supersonic jet expansion by resonant two-photon ionization. 1-Phenylimidazole is reported to be inhibitor of calmodulin-dependent nitric-oxide synthase from bovine brain and GHs pituitary cells.
Aplicación
1-Phenylimidazole is a suitable reagent used to investigate its effect on the citrulline formation by bovine brain nitric-oxide synthase.
Palabra de señalización
Warning
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
235.4 °F - closed cup
Punto de inflamabilidad (°C)
113 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
J A Zijlstra et al.
Mutation research, 198(1), 73-83 (1988-03-01)
The route of administration of a drug is a pharmacological factor to be reckoned with. In Drosophila, a whole-animal object for mutagenicity studies, the way in which a mutagen is applied can also be of crucial importance. In this study
Priyadarshini Balaraman et al.
Biochimica et biophysica acta. General subjects, 1863(2), 304-312 (2018-11-06)
The camphor-degrading microorganism, Pseudomonas putida strain ATCC 17453, is an aerobic, gram-negative soil bacterium that uses camphor as its sole carbon and energy source. The genes responsible for the catabolic degradation of camphor are encoded on the extra-chromosomal CAM plasmid.
Active-site structure analysis of recombinant human inducible nitric oxide synthase using imidazole.
R M Chabin et al.
Biochemistry, 35(29), 9567-9575 (1996-07-23)
Nitric oxide synthase catalyzes the pyridine nucleotide-dependent oxidation of L-arginine to nitric oxide and L-citrulline. It is a specialized cytochrome P450 monooxygenase that is sensitive to inhibition by imidazole. Steady-state kinetic studies on recombinant human inducible nitric oxide synthase (rH-iNOS)
Evan G Robertson et al.
The Journal of chemical physics, 121(24), 12421-12427 (2004-12-21)
The S(1)<--S(0) transition of 1-phenylimidazole (1PI) has been studied in a supersonic jet expansion by resonant two-photon ionization. The origin band at 36 075 cm(-1) is accompanied by a low frequency progression associated with torsion about the bond connecting phenyl
A R Barros et al.
Mutation research, 321(3), 119-126 (1994-05-01)
In order to investigate the role of metabolism in acrolein genotoxicity in D. melanogaster, the action of several metabolism modifiers, namely phenobarbital, an inducer of xenobiotic metabolism, phenylimidazole and iproniazid, inhibitors of oxidative activities of cytochrome P450, and diethyl maleate
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico











![1-[2-(Trifluoromethyl)phenyl]imidazole](/deepweb/assets/sigmaaldrich/product/structures/150/780/ea7e6b25-7659-422e-868c-8df7fd70d66e/640/ea7e6b25-7659-422e-868c-8df7fd70d66e.png)

