328014
3-Methylhippuric acid
98%
Sinónimos:
N-(3-Methylbenzoyl)glycine, m-Toluric acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
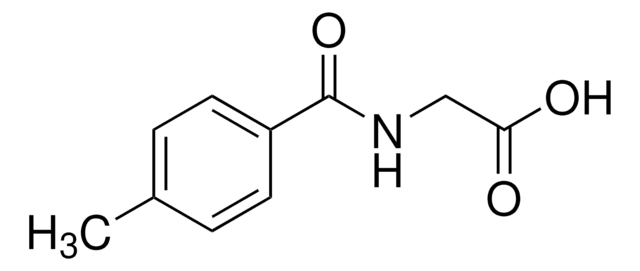
CH3C6H4CONHCH2CO2H
Número de CAS:
Peso molecular:
193.20
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
98%
mp
138-140 °C (lit.)
grupo funcional
amide
carboxylic acid
cadena SMILES
Cc1cccc(c1)C(=O)NCC(O)=O
InChI
1S/C10H11NO3/c1-7-3-2-4-8(5-7)10(14)11-6-9(12)13/h2-5H,6H2,1H3,(H,11,14)(H,12,13)
Clave InChI
YKAKNMHEIJUKEX-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
3-Methylhippuric acid is also referred as m-methyl-hippuric acid. It is major product of xylene biotransformation in urine.
Aplicación
3-Methylhippuric acid was employed as biological marker in studies on occupational exposure to xylene (solvent).
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
D de Carvalho et al.
International archives of occupational and environmental health, 63(1), 33-37 (1991-01-01)
The industrial solvents, toluene and xylene, have physicochemical properties that can be hazardous to the workers exposed. Since hippuric acid and m-methyl-hippuric acid represent the products of toluene and xylene biotransformation in urine, they are used as biological markers in
L Campbell et al.
British journal of industrial medicine, 45(2), 127-132 (1988-02-01)
In a series of experiments to investigate interactions between industrial solvents and common medications the interaction between m-xylene and aspirin was studied. As both these substances are metabolised and excreted as glycine conjugates there would possibly be competition for this
R Tardif et al.
Occupational and environmental medicine, 51(3), 187-191 (1994-03-01)
This study was undertaken to determine whether previous subacute treatment with ethanol could modify the kinetics of m-xylene in humans. A group of six volunteers was exposed twice to either 100 or 400 ppm of m-xylene during two hours (between
A Astier
Journal of chromatography, 573(2), 318-322 (1992-01-17)
A high-performance liquid chromatographic method is described for the simultaneous determination of six urinary metabolites of several aromatic chemicals: phenol (from benzene), hippuric acid (from toluene), 3-methylhippuric acid (from xylene), mandelic and phenylglyoxylic acid (from styrene) and 4-nitrophenol (from nitrobenzene).
Possible preferential metabolism of xylene isomers following occupational exposure to mixed xylenes.
M J Miller et al.
International archives of occupational and environmental health, 72(2), 89-97 (1999-04-10)
Solvent exposures commonly involve mixtures of substances or mixtures of isomers of a single solvent. These may be metabolised through common pathways, resulting in the potential for metabolic interactions. These may then lead to accumulation of solvent or metabolic intermediates
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico