8.52064
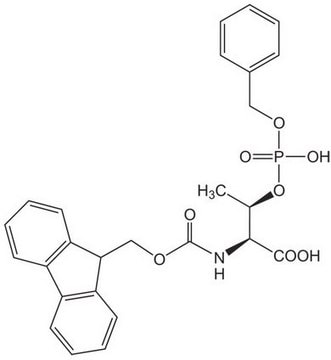
Fmoc-(FmocHmb)Gly-OH
Novabiochem®
Synonyme(s) :
Fmoc-(FmocHmb)Gly-OH, N-α-Fmoc-N-α-(2-Fmoc-oxy-4-methoxybenzyl)-glycine
About This Item
Produits recommandés
Niveau de qualité
Gamme de produits
Novabiochem®
Pureté
≥95.0% (acidimetric)
≥98% (TLC)
≥98.0% (HPLC)
Forme
powder
Capacité de réaction
reaction type: Fmoc solid-phase peptide synthesis
Fabricant/nom de marque
Novabiochem®
Application(s)
peptide synthesis
Groupe fonctionnel
Fmoc
Température de stockage
2-8°C
Description générale
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] T. Johnson, et al. (1993) J. Chem. Soc., Chem. Commun., 369.
[2] C. Hyde, et al. (1994) Int. J. Peptide Protein Res., 43, 431.
[3] L. C. Packman, et al. (1994) Pept. Res., 7, 125.
[4] T. Johnson, et al. (1994) Tetrahedron Lett., 35, 463.
[5] R. G. Simmonds (1996) Int. J. Peptide Protein Res., 47, 36.
[6] T. Johnson, et al. (1995) Lett. Pept. Sci., 1, 11.
[7] M. Quibell, et al. (1995) J. Am. Chem. Soc., 117, 11656.
[8] M. Quibell, et al. (1996) J. Chem. Soc., Perkin Trans. 1, 1227.
[9] M. Quibell, et al. (1994) Tetrahedron Lett., 35, 2237.
[10] M. Quibell, et al. (1994) J. Org. Chem., 59, 1745.
[11] M. Quibell, et al. (1995) J. Chem. Soc., Perkin Trans. 1, 2019.
[12] M. Quibell, et al. (1994) J. Chem. Soc., Chem. Commun., 2343.
[13] L. C. Packman (1995) Tetrahedron Lett., 36, 7523.
[14] J. Offer, et al. (1996) J. Chem. Soc., Perkin Trans. 1, 175.
[15] W. R. Sampson, et al. (1999) J. Peptide Sci., 5, 403.
Application
- Advances in Fmoc solid‐phase peptide synthesis: An overview of recent advancements in Fmoc-SPPS, including the use of specialized Fmoc-amino acids like Fmoc-(FmocHmb)Gly-OH to improve synthesis efficiency and outcomes (J Offer et al., 2016).
Liaison
Remarque sur l'analyse
Appearance of substance (visual): powder
Identity (IR): passes test
Purity (TLC(157A)): ≥ 98 %
Purity (TLC(157B)): ≥ 98 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 95.0 %
Water (K. F.): ≤ 1.00 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Protocoles
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique