8.51086
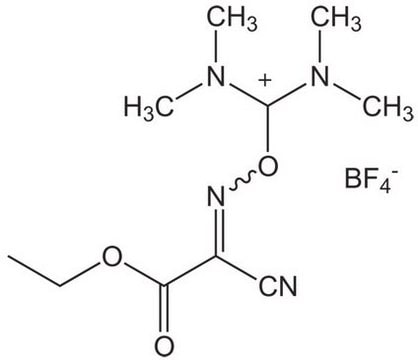
Oxyma Pure
≥99.0% (HPLC), for peptide synthesis, Novabiochem®
Synonyme(s) :
Oxyma Pure, Ethyl cyano(hydroxyimino)acetate
About This Item
Produits recommandés
product name
Oxyma Pure, Novabiochem®
Niveau de qualité
Gamme de produits
Novabiochem®
Pureté
≥99.0% (HPLC)
Forme
crystalline powder
Capacité de réaction
reaction type: Coupling Reactions
Fabricant/nom de marque
Novabiochem®
Pf
130-132 °C
Application(s)
peptide synthesis
Température de stockage
2-8°C
InChI
1S/C5H6N2O3/c1-2-10-5(8)4(3-6)7-9/h9H,2H2,1H3/b7-4+
Clé InChI
LCFXLZAXGXOXAP-QPJJXVBHSA-N
Description générale
The generation of toxic hydrogen cyanide (HCN) from the reaction between Oxyma Pure and DIC has been observed. Fortunately, this side reaction can be eliminated by substituting DIC for the more hindered t-butylethylcarbodiimide.
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents
Literature references:
[1] R. Subirós-Funosas, et al. (2009) Chem. Eur. J., 15, 9394
[2] J. Collins , et al. (2014) Org . Lett ., 16, 940.
[3] A. D. McFarland (2019) Org. Process Res. Dev., 23, 2099.
[4] S. R. Manne, et al (2022) Org. Process Res. Dev., 26, 2894.
Application
- Use in the synthesis of difficult peptides.
- As an acidic modifier to prevent aspartimide formation.
Caractéristiques et avantages
- Can be used in place of HOBt in carbodiimide-mediated coupling reactions without change of protocol
- It gives results comparable to HOAt in step-wise solid-phase synthesis
- Less epimerization than HOBt in fragment condensation reactions
Remarque sur l'analyse
Appearance of substance (visual): crystalline powder
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Identity (IR): passes test
Solubility (12,5 mmol in 25 ml DMF): clearly soluble
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique